Mechanical and Antimicrobial Polyethylene Composites with CaO Nanoparticles
- PMID: 32961957
- PMCID: PMC7570308
- DOI: 10.3390/polym12092132
Mechanical and Antimicrobial Polyethylene Composites with CaO Nanoparticles
Abstract
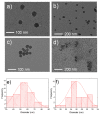
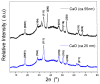
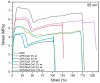
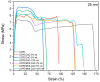
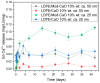
Low-density polyethylene composites containing different sizes of calcium oxide (CaO) nanoparticles were obtained by melt mixing. The CaO nanoparticles were synthesized by either the sol-gel or sonication methods, obtaining two different sizes: ca. 55 nm and 25 nm. These nanoparticles were used either as-synthesized or were modified organically on the surface with oleic acid (Mod-CaO), at concentrations of 3, 5, and 10 wt% in the polymer. The Mod-CaO nanoparticles of 25 nm can act as nucleating agents, increasing the polymer's crystallinity. The Young's Modulus increased with the Mod-CaO nanoparticles, rendering higher reinforcement effects with an increase as high as 36%. The reduction in Escherichia coli bacteria in the nanocomposites increased with the amount of CaO nanoparticles, the size reduction, and the surface modification. The highest antimicrobial behavior was found in the composites with a Mod-CaO of 25 nm, presenting a reduction of 99.99%. This strong antimicrobial effect can be associated with the release of the Ca2+ from the composites, as studied for the composite with 10 wt% nanoparticles. The ion release was dependent on the size of the nanoparticles and their surface modification. These findings show that CaO nanoparticles are an excellent alternative as an antimicrobial filler in polymer nanocomposites to be applied for food packaging or medical devices.
Keywords: CaO nanoparticles; biocidal activity; ion release (Ca2+); mechanical properties; nanocomposite; polyethylene matrix.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Wang Q., Zhou Z., Song L., Xu H., Wang L. Nanoscopic confinement effects on ethylene polymerization by intercalated silicate with metallocene catalyst. J. Polym. Sci. Pol. Chem. 2004;42:38–43. doi: 10.1002/pola.10987. - DOI
-
- Hwu J., Jiang G. Preparation and characterization of polypropylene-montmorillonite nanocomposites generated by in situ metallocene catalyst polymerization. J. Appl. Polym. Sci. 2005;95:1228–1236. doi: 10.1002/app.21325. - DOI
-
- Paiva L.B., Morales A.R., Valenzuela Díaz F.R. Organoclays: Properties, preparation and applications. Appl. Clay Sci. 2008;42:8–24. doi: 10.1016/j.clay.2008.02.006. - DOI
-
- Zapata P.A., Quijada R., Covarrubias C., Moncada E., Retuert J. Catalytic activity during the preparation of PE/clay nanocomposites by in situ polymerization with metallocene catalysts. J. Appl. Polym. Sci. 2009;113:2368–2377. doi: 10.1002/app.30334. - DOI
-
- Pavlidou S., Papaspyrides C.D. A review on polymer-layered silicate nanocomposites. Prog. Polym. Sci. 2008;33:1119–1198. doi: 10.1016/j.progpolymsci.2008.07.008. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

