Reelin Mediates Hippocampal Cajal-Retzius Cell Positioning and Infrapyramidal Blade Morphogenesis
- PMID: 32962021
- PMCID: PMC7558149
- DOI: 10.3390/jdb8030020
Reelin Mediates Hippocampal Cajal-Retzius Cell Positioning and Infrapyramidal Blade Morphogenesis
Abstract
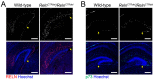
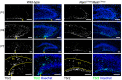
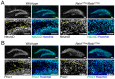
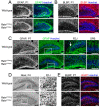
We have previously described hypomorphic reelin (Reln) mutant mice, RelnCTRdel, in which the morphology of the dentate gyrus is distinct from that seen in reeler mice. In the RelnCTRdel mutant, the infrapyramidal blade of the dentate gyrus fails to extend, while the suprapyramidal blade forms with a relatively compact granule neuron layer. Underlying this defect, we now report several developmental anomalies in the RelnCTRdel dentate gyrus. Most strikingly, the distribution of Cajal-Retzius cells was aberrant; Cajal-Retzius neurons were increased in the suprapyramidal blade, but were greatly reduced along the subpial surface of the prospective infrapyramidal blade. We also observed multiple abnormalities of the fimbriodentate junction. Firstly, progenitor cells were distributed abnormally; the "neurogenic cluster" at the fimbriodentate junction was absent, lacking the normal accumulation of Tbr2-positive intermediate progenitors. However, the number of dividing cells in the dentate gyrus was not generally decreased. Secondly, a defect of secondary glial scaffold formation, limited to the infrapyramidal blade, was observed. The densely radiating glial fibers characteristic of the normal fimbriodentate junction were absent in mutants. These fibers might be required for migration of progenitors, which may account for the failure of neurogenic cluster formation. These findings suggest the importance of the secondary scaffold and neurogenic cluster of the fimbriodentate junction in morphogenesis of the mammalian dentate gyrus. Our study provides direct genetic evidence showing that normal RELN function is required for Cajal-Retzius cell positioning in the dentate gyrus, and for formation of the fimbriodentate junction to promote infrapyramidal blade extension.
Keywords: dentate gyrus; hippocampus; migration; neurogenesis; postnatal development; reelin protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
p73 and Reelin in Cajal-Retzius cells of the developing human hippocampal formation.Cereb Cortex. 2004 May;14(5):484-95. doi: 10.1093/cercor/bhh010. Epub 2004 Mar 28. Cereb Cortex. 2004. PMID: 15054064
-
Development of afferent fiber lamination in the infrapyramidal blade of the rat dentate gyrus.J Comp Neurol. 1999 Aug 23;411(2):257-66. J Comp Neurol. 1999. PMID: 10404251
-
Tbr2 expression in Cajal-Retzius cells and intermediate neuronal progenitors is required for morphogenesis of the dentate gyrus.J Neurosci. 2013 Feb 27;33(9):4165-80. doi: 10.1523/JNEUROSCI.4185-12.2013. J Neurosci. 2013. PMID: 23447624 Free PMC article.
-
Cajal, Retzius, and Cajal-Retzius cells.Front Neuroanat. 2014 Jun 17;8:48. doi: 10.3389/fnana.2014.00048. eCollection 2014. Front Neuroanat. 2014. PMID: 24987337 Free PMC article. Review.
-
Dual role of Cajal-Retzius cells and reelin in cortical development.Cell Tissue Res. 1997 Nov;290(2):315-22. doi: 10.1007/s004410050936. Cell Tissue Res. 1997. PMID: 9321693 Review.
Cited by
-
Monoallelic and biallelic mutations in RELN underlie a graded series of neurodevelopmental disorders.Brain. 2022 Sep 14;145(9):3274-3287. doi: 10.1093/brain/awac164. Brain. 2022. PMID: 35769015 Free PMC article.
-
Special Issue "2020 Feature Papers by JDB' Editorial Board Members".J Dev Biol. 2021 Jun 2;9(2):21. doi: 10.3390/jdb9020021. J Dev Biol. 2021. PMID: 34199485 Free PMC article.
-
Lmx1a is a master regulator of the cortical hem.Elife. 2023 Sep 19;12:e84095. doi: 10.7554/eLife.84095. Elife. 2023. PMID: 37725078 Free PMC article.
References
-
- Hodge R.D., Garcia A.J., Elsen G.E., Nelson B.R., Mussar K.E., Reiner S.L., Ramirez J.-M., Hevner R.F. Tbr2 Expression in Cajal-Retzius Cells and Intermediate Neuronal Progenitors Is Required for Morphogenesis of the Dentate Gyrus. J. Neurosci. 2013;33:4165–4180. doi: 10.1523/JNEUROSCI.4185-12.2013. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

