Preventive Triple Gene Therapy Reduces the Negative Consequences of Ischemia-Induced Brain Injury after Modelling Stroke in a Rat
- PMID: 32962079
- PMCID: PMC7558841
- DOI: 10.3390/ijms21186858
Preventive Triple Gene Therapy Reduces the Negative Consequences of Ischemia-Induced Brain Injury after Modelling Stroke in a Rat
Abstract
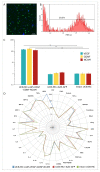
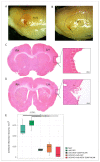
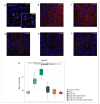
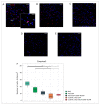
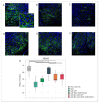
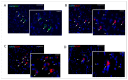
Currently, the main fundamental and clinical interest for stroke therapy is focused on developing a neuroprotective treatment of a penumbra region within the therapeutic window. The development of treatments for ischemic stroke in at-risk patients is of particular interest. Preventive gene therapy may significantly reduce the negative consequences of ischemia-induced brain injury. In the present study, we suggest the approach of preventive gene therapy for stroke. Adenoviral vectors carrying genes encoding vascular endothelial growth factor (VEGF), glial cell-derived neurotrophic factor (GDNF) and neural cell adhesion molecule (NCAM) or gene engineered umbilical cord blood mononuclear cells (UCB-MC) overexpressing recombinant VEGF, GDNF, and NCAM were intrathecally injected before distal occlusion of the middle cerebral artery in rats. Post-ischemic brain recovery was investigated 21 days after stroke modelling. Morphometric and immunofluorescent analysis revealed a reduction of infarction volume accompanied with a lower number of apoptotic cells and decreased expression of Hsp70 in the peri-infarct region in gene-treated animals. The lower immunopositive areas for astrocytes and microglial cells markers, higher number of oligodendrocytes and increased expression of synaptic proteins suggest the inhibition of astrogliosis, supporting the corresponding myelination and functional recovery of neurons in animals receiving preventive gene therapy. In this study, for the first time, we provide evidence of the beneficial effects of preventive triple gene therapy by an adenoviral- or UCB-MC-mediated intrathecal simultaneous delivery combination of vegf165, gdnf, and ncam1 on the preservation and recovery of the brain in rats with subsequent modelling of stroke.
Keywords: GDNF; NCAM; VEGF; adenoviral vector; human umbilical cord blood mononuclear cells; preventive gene therapy; stroke.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
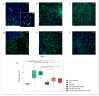
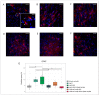
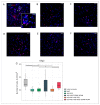
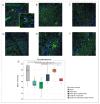

References
-
- Karlupia N., Manley N.C., Prasad K., Schäfer R., Steinberg G.K. Intraarterial transplantation of human umbilical cord blood mononuclear cells is more efficacious and safer compared with umbilical cord mesenchymal stromal cells in a rodent stroke model. Stem Cell Res. Ther. 2014;5:45. doi: 10.1186/scrt434. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

