E4 Transcription Factor 1 (E4F1) Regulates Sertoli Cell Proliferation and Fertility in Mice
- PMID: 32962114
- PMCID: PMC7552733
- DOI: 10.3390/ani10091691
E4 Transcription Factor 1 (E4F1) Regulates Sertoli Cell Proliferation and Fertility in Mice
Abstract
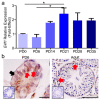
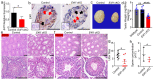
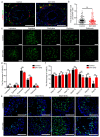
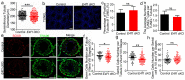
In the mammalian testes, Sertoli cells are the only somatic cells in the seminiferous tubules that provide structural, nutritional and regulatory support for developing spermatogenic cells. Sertoli cells only proliferate during the fetal and neonatal periods and enter a quiescent state after puberty. Functional evidences suggest that the size of Sertoli cell population determines sperm production and fertility. However, factors that direct Sertoli cell proliferation and maturation are not fully understood. Transcription factor E4F1 is a multifunctional protein that serves essential roles in cell fate decisions and because it interacts with pRB, a master regulator of Sertoli cell function, we hypothesized that E4F1 may have a functional role in Sertoli cells. E4f1 mRNA was present in murine testis and immunohistochemical staining confirmed that E4F1 was enriched in mature Sertoli cells. We generated a conditional knockout mouse model using Amh-cre and E4f1flox/flox lines to study E4F1 fucntion in Sertoli cells and the results showed that E4f1 deletion caused a significant reduction in testis size and fertility. Further analyses revealed that meiosis progression and spermiogenesis were normal, however, Sertoli cell proliferation was impaired and germ cell apoptosis was elevated in the testis of E4f1 conditional knockout mice. On the basis of these findings, we concluded that E4F1 was expressed in murine Sertoli cells and served important functions in regulating Sertoli cell proliferation and fertility.
Keywords: E4F1; fertility; proliferation; sertoli cells; spermatogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Germ cell-specific disruption of the Meig1 gene causes impaired spermiogenesis in mice.Andrology. 2013 Jan;1(1):37-46. doi: 10.1111/j.2047-2927.2012.00001.x. Epub 2012 Aug 30. Andrology. 2013. PMID: 23258628 Free PMC article.
-
Npat-dependent programmed Sertoli cell proliferation is indispensable for testis cord development and germ cell mitotic arrest.FASEB J. 2019 Aug;33(8):9075-9086. doi: 10.1096/fj.201802289RR. Epub 2019 May 14. FASEB J. 2019. PMID: 31084574
-
Specific deletion of Cdh2 in Sertoli cells leads to altered meiotic progression and subfertility of mice.Biol Reprod. 2015 Mar;92(3):79. doi: 10.1095/biolreprod.114.126334. Epub 2015 Jan 28. Biol Reprod. 2015. PMID: 25631347
-
Ontogeny of the androgen receptor expression in the fetal and postnatal testis: its relevance on Sertoli cell maturation and the onset of adult spermatogenesis.Microsc Res Tech. 2009 Nov;72(11):787-95. doi: 10.1002/jemt.20754. Microsc Res Tech. 2009. PMID: 19551717 Review.
-
Spermatogenesis in nonmammalian vertebrates.Microsc Res Tech. 1995 Dec 15;32(6):459-97. doi: 10.1002/jemt.1070320602. Microsc Res Tech. 1995. PMID: 8605396 Review.
Cited by
-
Cellular and Molecular Insights Into the Etiology of Subfertility/Infertility in Crossbred Bulls (Bos taurus × Bos indicus): A Review.Front Cell Dev Biol. 2021 Jul 8;9:696637. doi: 10.3389/fcell.2021.696637. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34307374 Free PMC article. Review.
-
[Research progress on glycolipid metabolism of Sertoli cell in the development of spermatogenic cell].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2025 Mar 25;54(2):257-265. doi: 10.3724/zdxbyxb-2024-0346. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40065698 Free PMC article. Review. Chinese.
-
Transcription factor E4F1 as a regulator of cell life and disease progression.Sci Adv. 2023 Sep 29;9(39):eadh1991. doi: 10.1126/sciadv.adh1991. Epub 2023 Sep 29. Sci Adv. 2023. PMID: 37774036 Free PMC article. Review.
-
Multi-Level Control of the ATM/ATR-CHK1 Axis by the Transcription Factor E4F1 in Triple-Negative Breast Cancer.Int J Mol Sci. 2022 Aug 16;23(16):9217. doi: 10.3390/ijms23169217. Int J Mol Sci. 2022. PMID: 36012478 Free PMC article.
-
A single-nucleus transcriptomic atlas of primate testicular aging reveals exhaustion of the spermatogonial stem cell reservoir and loss of Sertoli cell homeostasis.Protein Cell. 2023 Dec 1;14(12):888-907. doi: 10.1093/procel/pwac057. Protein Cell. 2023. PMID: 36929025 Free PMC article.
References
-
- Hess R.A., Franca L.R.D. Spermatogenesis and Cycle of the Seminiferous Epithelium. Adv. Exp. Med. Biol. 2008;636:1. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

