NIR-Triggered Hyperthermal Effect of Polythiophene Nanoparticles Synthesized by Surfactant-Free Oxidative Polymerization Method on Colorectal Carcinoma Cells
- PMID: 32962169
- PMCID: PMC7564425
- DOI: 10.3390/cells9092122
NIR-Triggered Hyperthermal Effect of Polythiophene Nanoparticles Synthesized by Surfactant-Free Oxidative Polymerization Method on Colorectal Carcinoma Cells
Abstract
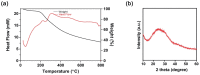
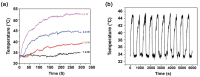
In this work, polythiophene nanoparticles (PTh-NPs) were synthesized by a surfactant-free oxidative chemical polymerization method at 60 °C, using ammonium persulphate as an oxidant. Various physicochemical properties were studied in terms of field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), Fourier transform infra-red (FT-IR) spectroscopy, and differential scanning calorimetry (DSC)/thermogravimetric analysis (TGA). Photothermal performance of the as-synthesized PTh-NPs was studied by irradiating near infra-red of 808 nm under different concentration of the substrate and power supply. The photothermal stability of PTh-NPs was also studied. Photothermal effects of the as-synthesized PTh-NPs on colorectal cancer cells (CT-26) were studied at 100 µg/mL concentration and 808 nm NIR irradiation of 2.0 W/cm2 power. Our in vitro results showed remarkable NIR laser-triggered photothermal apoptotic cell death by PTh-NPs. Based on the experimental findings, it is revealed that PTh-NPs can act as a heat mediator and can be an alternative material for photothermal therapy in cancer treatment.
Keywords: CT-26 cells; apoptotic cell death; cancer treatment; chemical polymerization; photothermal; polythiophene nanoparticles.
Conflict of interest statement
The author declares no conflict of interest.
Figures




Similar articles
-
Sacrificial template-based synthetic approach of polypyrrole hollow fibers for photothermal therapy.J Colloid Interface Sci. 2019 Jan 15;534:447-458. doi: 10.1016/j.jcis.2018.09.047. Epub 2018 Sep 17. J Colloid Interface Sci. 2019. PMID: 30248614
-
Precisely Tuning Photothermal and Photodynamic Effects of Polymeric Nanoparticles by Controlled Copolymerization.Angew Chem Int Ed Engl. 2020 Jul 27;59(31):12756-12761. doi: 10.1002/anie.202004181. Epub 2020 May 25. Angew Chem Int Ed Engl. 2020. PMID: 32343868
-
Semiconducting Polycomplex Nanoparticles for Photothermal Ferrotherapy of Cancer.Angew Chem Int Ed Engl. 2020 Jun 22;59(26):10633-10638. doi: 10.1002/anie.202003004. Epub 2020 Apr 20. Angew Chem Int Ed Engl. 2020. PMID: 32207214
-
Semiconducting polymer-based nanoparticles for photothermal therapy at the second near-infrared window.Chem Commun (Camb). 2018 Dec 14;54(96):13599-13602. doi: 10.1039/c8cc07583b. Epub 2018 Nov 19. Chem Commun (Camb). 2018. PMID: 30451251
-
Polydopamine-Based Nanoparticles for Photothermal Therapy/Chemotherapy and their Synergistic Therapy with Autophagy Inhibitor to Promote Antitumor Treatment.Chem Rec. 2021 Apr;21(4):781-796. doi: 10.1002/tcr.202000170. Epub 2021 Feb 26. Chem Rec. 2021. PMID: 33634962 Review.
Cited by
-
Metal oxide-polymer hybrid composites: a comprehensive review on synthesis and multifunctional applications.RSC Adv. 2025 Jun 2;15(23):18173-18208. doi: 10.1039/d5ra01821h. eCollection 2025 May 29. RSC Adv. 2025. PMID: 40458437 Free PMC article. Review.
-
Improved lymph node detection in minimally invasive radical surgery for colorectal cancer using nanocarbon tracer.Discov Oncol. 2024 Nov 28;15(1):720. doi: 10.1007/s12672-024-01582-0. Discov Oncol. 2024. PMID: 39609297 Free PMC article.
-
Clinical value of nano-carbon lymphatic tracer for regional lymph node dissections of rectal cancer after neoadjuvant chemoradiotherapy.J Appl Clin Med Phys. 2024 Aug;25(8):e14406. doi: 10.1002/acm2.14406. Epub 2024 May 31. J Appl Clin Med Phys. 2024. PMID: 38820538 Free PMC article.
-
Current Landscape in Organic Nanosized Materials Advances for Improved Management of Colorectal Cancer Patients.Materials (Basel). 2021 May 8;14(9):2440. doi: 10.3390/ma14092440. Materials (Basel). 2021. PMID: 34066710 Free PMC article. Review.
-
Recent Progress in Metal-Organic Framework-Derived Nanostructures in the Removal of Volatile Organic Compounds.Molecules. 2021 Aug 16;26(16):4948. doi: 10.3390/molecules26164948. Molecules. 2021. PMID: 34443537 Free PMC article. Review.
References
-
- Pal I., Rajesh Y., Banik P., Dey G., Dey K.K., Bharti R., Naskar D., Chakraborty S., Ghosh S.K., Das S.K., et al. Prevention of epithelial to mesenchymal transition in colorectal carcinoma by regulation of the E-cadherin-β-catenin-vinculin axis. Cancer Lett. 2019;452:254–263. doi: 10.1016/j.canlet.2019.03.008. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

