Freeze-Drying of Platelet-Rich Plasma: The Quest for Standardization
- PMID: 32962283
- PMCID: PMC7555364
- DOI: 10.3390/ijms21186904
Freeze-Drying of Platelet-Rich Plasma: The Quest for Standardization
Abstract
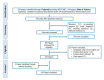
The complex biology of platelets and their involvement in tissue repair and inflammation have inspired the development of platelet-rich plasma (PRP) therapies for a broad array of medical needs. However, clinical advances are hampered by the fact that PRP products, doses and treatment protocols are far from being standardized. Freeze-drying PRP (FD-PRP) preserves platelet function, cytokine concentration and functionality, and has been proposed as a consistent method for product standardization and fabrication of an off-the-shelf product with improved stability and readiness for future uses. Here, we present the current state of experimental and clinical FD-PRP research in the different medical areas in which PRP has potential to meet prevailing medical needs. A systematic search, according to PRISMA (Preferred Reported Items for Systematic Reviews and Meta-Analyses) guidelines, showed that research is mostly focused on wound healing, i.e., developing combination products for ulcer management. Injectable hydrogels are investigated for lumbar fusion and knee conditions. In dentistry, combination products permit slow kinetics of growth factor release and functionalized membranes for guided bone regeneration.
Keywords: dentistry; fibrin; freeze-drying; orthopedics; platelet-rich plasma; wound healing.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

