Coordination Mechanism and Bio-Evidence: Reactive γ-Ketoenal Intermediated Hepatotoxicity of Psoralen and Isopsoralen Based on Computer Approach and Bioassay
- PMID: 32962321
- PMCID: PMC6151710
- DOI: 10.3390/molecules22091451
Coordination Mechanism and Bio-Evidence: Reactive γ-Ketoenal Intermediated Hepatotoxicity of Psoralen and Isopsoralen Based on Computer Approach and Bioassay
Abstract
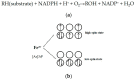
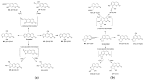
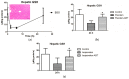
Psoralen and isopsoralen are secondary plant metabolites found in many fruits, vegetables, and medicinal herbs. Psoralen-containing plants (Psoralea corylifolia L.) have been reported to cause hepatotoxicity. Herein, we found that psoralen and isopsoralen were oxidized by CYP450s to reactive furanoepoxide or γ-ketoenal intermediates, causing a mechanism-based inhibition of CYP3A4. Furthermore, in GSH-depleted mice, the hepatotoxicity of these reactive metabolites has been demonstrated by pre-treatment with a well-known GSH synthesis inhibitor, L-buthionine-S, Rsulfoxinine (BSO). Moreover, a molecular docking simulation of the present study was undertaken to understand the coordination reaction that plays a significant role in the combination of unstable intermediates and CYP3A4. These results suggested that psoralen and isopsoralen are modest hepatotoxic agents, as their reactive metabolites could be deactivated by H2O and GSH in the liver, which partly contributes to the ingestion of psoralen-containing fruits and vegetables being safe.
Keywords: CYP3A4; GSH depletion; coordination compound; furanoepoxide; hepatoxicity; molecular docking; γ-ketoenal intermediate.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures









References
-
- Conforti F., Marrelli M., Menichini F., Bonesi M., Statti G., Provenzano E., Menichini F. Natural and synthetic furanocoumarins as treatment for vitiligo and psoriasis. Curr. Drug Ther. 2009;4:38–58. doi: 10.2174/157488509787081886. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

