Meta-analysis of diagnostic performance of serology tests for COVID-19: impact of assay design and post-symptom-onset intervals
- PMID: 32962560
- PMCID: PMC7580610
- DOI: 10.1080/22221751.2020.1826362
Meta-analysis of diagnostic performance of serology tests for COVID-19: impact of assay design and post-symptom-onset intervals
Abstract
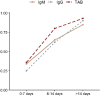
Serology detection is recognized for its sensitivity in convalescent patients with COVID-19, in comparison with nucleic acid amplification tests (NAATs). This article aimed to evaluate the diagnostic accuracy of serologic methods for COVID-19 based on assay design and post-symptom-onset intervals. Two authors independently searched PubMed, Cochrane library, Ovid, EBSCO for case-control, longitudinal and cohort studies that determined the diagnostic accuracy of serology tests in comparison with NAATs in COVID-19 cases and used QUADAS-2 for quality assessment. Pooled accuracy was analysed using INLA method. A total of 27 studies were included in this meta-analysis, with 4 cohort, 16 case-control and 7 longitudinal studies and 4565 participants. Serology tests had the lowest sensitivity at 0-7 days after symptom onset and the highest at >14 days. TAB had a better sensitivity than IgG or IgM only. Using combined nucleocapsid (N) and spike(S) protein had a better sensitivity compared to N or S protein only. Lateral flow immunoassay (LFIA) had a lower sensitivity than enzyme-linked immunoassay (ELISA) and chemiluminescent immunoassay (CLIA). Serology tests will play an important role in the clinical diagnosis for later stage COVID-19 patients. ELISA tests, detecting TAB or targeting combined N and S proteins had a higher diagnostic sensitivity compared to other methods.
Keywords: COVID-19; SARS-CoV-2; immunoassays; metanalysis; serology.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
MJL and YWT are employees of Cepheid, the commercial manufacturer of the Xpert Xpress SARS-CoV-2 test. HYW, JWA and WHZ declare no competing interests.
Figures



References
-
- World Health Organizaiton . Coronavirus disease 2019 (COVID-19) Situation Report – 51 [Internet]. 2020 [cited 2020 Jul 10]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2....
-
- World Health Organization. Coronavirus diseases . (2019). (COVID-19) Situation Report-172 [Internet]. 2020 [cited 2020 Jul 10]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2....
-
- Office of the State Administration of Traditional Chinese Medicine, National Health Commission . Novel Coronavirus Pneumonia (trial version 7) [Internet]. 2020 [cited 2020 Jul 10]; Available from: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb19....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
