Multicentre study of 4626 patients assesses the effectiveness, safety and burden of two categories of treatments for central retinal vein occlusion: intravitreal anti-vascular endothelial growth factor injections and intravitreal Ozurdex injections
- PMID: 32962992
- PMCID: PMC8140590
- DOI: 10.1136/bjophthalmol-2020-317306
Multicentre study of 4626 patients assesses the effectiveness, safety and burden of two categories of treatments for central retinal vein occlusion: intravitreal anti-vascular endothelial growth factor injections and intravitreal Ozurdex injections
Abstract
Background/aims: To assess the effectiveness, burden and safety of two categories of treatment for central retinal vein occlusion (CRVO): intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) and dexamethasone (Ozurdex).
Methods: A retrospective analysis of Medisoft electronic medical record (EMR) data from 27 National Health Service sites in the UK identified 4626 treatment-naive patients with a single mode of treatment for macular oedema secondary to CRVO. Statistics describing the overall CRVO patient cohort and individual patient subpopulations stratified by treatment type were generated. Mean age at baseline, gender, ethnicity, social deprivation and visual acuity (VA) follow-up was reported. Absolute and change in VA using ETDRS are used to describe treatment effectiveness, the number of injections and visits used to describe treatment burden and endophthalmitis rates as a marker of treatment safety.
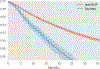
Results: Mean VA was 47.9 and 45.3 EDTRS letters in the anti-VEGF and Ozurdex groups, respectively. This changed to 57.9/53.7 at 12 months, 58.3/46.9 at 18 months and 59.4/51.0 at 36 months. Mean number of injections were 5.6/1.6 at 12 months, 6.0/1.7 at 18 months and 7.0/1.8 at 36 months. Endophthalmitis rates were 0.003% (n=4) for the anti-VEGF group and 0.09% (n=1) for the Ozurdex group.
Conclusions: VA improvements were greater and more sustained with anti-VEGF treatment. Lower starting acuity resulted in bigger gains in both groups, while higher starting acuity resulted in higher VA at 36 months. Although treatment burden was greater with anti-VEGF, Ozurdex was associated with higher rates of endophthalmitis.
Keywords: Macula; Retina; Treatment Medical; Vision.
© Author(s) (or their employer(s)) 2021. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PT received a grant from Novartis Pharmaceuticals. AT is a consultant to Allergan, Bayer, Heidelberg Engineering, Kanghong Pharmaceuticals, Novartis, Oxurion. CE is a consultant to Heidelberg Engineering. AL reports being an employee of the US Food and Drug Administration (US FDA), and received grants from Santen, Carl Zeiss Meditec and Novartis, personal fees from Genentech, Topcon and Verana Health, outside the submitted work. This article does not reflect the opinions of the US Government or of the US FDA.
Figures

References
-
- Clinical Guidelines Retinal Vein Occlusion (RVO) Guidelines.; 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
