Genotoxic Effect of Salmonella Paratyphi A Infection on Human Primary Gallbladder Cells
- PMID: 32963006
- PMCID: PMC7512552
- DOI: 10.1128/mBio.01911-20
Genotoxic Effect of Salmonella Paratyphi A Infection on Human Primary Gallbladder Cells
Abstract
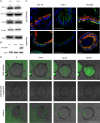
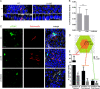
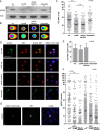
Carcinoma of the gallbladder (GBC) is the most frequent tumor of the biliary tract. Despite epidemiological studies showing a correlation between chronic infection with Salmonella enterica Typhi/Paratyphi A and GBC, the underlying molecular mechanisms of this fatal connection are still uncertain. The murine serovar Salmonella Typhimurium has been shown to promote transformation of genetically predisposed cells by driving mitogenic signaling. However, insights from this strain remain limited as it lacks the typhoid toxin produced by the human serovars Typhi and Paratyphi A. In particular, the CdtB subunit of the typhoid toxin directly induces DNA breaks in host cells, likely promoting transformation. To assess the underlying principles of transformation, we used gallbladder organoids as an infection model for Salmonella Paratyphi A. In this model, bacteria can invade epithelial cells, and we observed host cell DNA damage. The induction of DNA double-strand breaks after infection depended on the typhoid toxin CdtB subunit and extended to neighboring, non-infected cells. By cultivating the organoid derived cells into polarized monolayers in air-liquid interphase, we could extend the duration of the infection, and we observed an initial arrest of the cell cycle that does not depend on the typhoid toxin. Non-infected intoxicated cells instead continued to proliferate despite the DNA damage. Our study highlights the importance of the typhoid toxin in causing genomic instability and corroborates the epidemiological link between Salmonella infection and GBC.IMPORTANCE Bacterial infections are increasingly being recognized as risk factors for the development of adenocarcinomas. The strong epidemiological evidence linking Helicobacter pylori infection to stomach cancer has paved the way to the demonstration that bacterial infections cause DNA damage in the host cells, initiating transformation. In this regard, the role of bacterial genotoxins has become more relevant. Salmonella enterica serovars Typhi and Paratyphi A have been clinically associated with gallbladder cancer. By harnessing the stem cell potential of cells from healthy human gallbladder explant, we regenerated and propagated the epithelium of this organ in vitro and used these cultures to model S. Paratyphi A infection. This study demonstrates the importance of the typhoid toxin, encoded only by these specific serovars, in causing genomic instability in healthy gallbladder cells, posing intoxicated cells at risk of malignant transformation.
Keywords: DNA damage; Salmonella; gallbladder; gallbladder cancer; mucosoid cultures; organoid cultures; typhoid toxin.
Copyright © 2020 Sepe, Hartl et al.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

