Identification of the skeletal progenitor cells forming osteophytes in osteoarthritis
- PMID: 32963046
- PMCID: PMC8136618
- DOI: 10.1136/annrheumdis-2020-218350
Identification of the skeletal progenitor cells forming osteophytes in osteoarthritis
Abstract
Objectives: Osteophytes are highly prevalent in osteoarthritis (OA) and are associated with pain and functional disability. These pathological outgrowths of cartilage and bone typically form at the junction of articular cartilage, periosteum and synovium. The aim of this study was to identify the cells forming osteophytes in OA.
Methods: Fluorescent genetic cell-labelling and tracing mouse models were induced with tamoxifen to switch on reporter expression, as appropriate, followed by surgery to induce destabilisation of the medial meniscus. Contributions of fluorescently labelled cells to osteophytes after 2 or 8 weeks, and their molecular identity, were analysed by histology, immunofluorescence staining and RNA in situ hybridisation. Pdgfrα-H2BGFP mice and Pdgfrα-CreER mice crossed with multicolour Confetti reporter mice were used for identification and clonal tracing of mesenchymal progenitors. Mice carrying Col2-CreER, Nes-CreER, LepR-Cre, Grem1-CreER, Gdf5-Cre, Sox9-CreER or Prg4-CreER were crossed with tdTomato reporter mice to lineage-trace chondrocytes and stem/progenitor cell subpopulations.
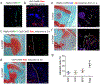
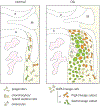
Results: Articular chondrocytes, or skeletal stem cells identified by Nes, LepR or Grem1 expression, did not give rise to osteophytes. Instead, osteophytes derived from Pdgfrα-expressing stem/progenitor cells in periosteum and synovium that are descendants from the Gdf5-expressing embryonic joint interzone. Further, we show that Sox9-expressing progenitors in periosteum supplied hybrid skeletal cells to the early osteophyte, while Prg4-expressing progenitors from synovial lining contributed to cartilage capping the osteophyte, but not to bone.
Conclusion: Our findings reveal distinct periosteal and synovial skeletal progenitors that cooperate to form osteophytes in OA. These cell populations could be targeted in disease modification for treatment of OA.
Keywords: arthritis; chondrocytes; experimental; fibroblasts; osteoarthritis.
© Author(s) (or their employer(s)) 2020. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





References
-
- van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthritis Cartilage 2007;15:237–44. - PubMed
-
- Gelse K, Söder S, Eger W, et al. Osteophyte development--molecular characterization of differentiation stages. Osteoarthritis Cartilage 2003;11:141–8. - PubMed
-
- Yamazaki S, Ema H, Karlsson G, et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 2011;147:1146–58. - PubMed
Publication types
MeSH terms
Grants and funding
- MC_PC_17230/MRC_/Medical Research Council/United Kingdom
- 26670/CRUK_/Cancer Research UK/United Kingdom
- T32 HD060549/HD/NICHD NIH HHS/United States
- MR/L020211/1/MRC_/Medical Research Council/United Kingdom
- C61367/A26670/CRUK_/Cancer Research UK/United Kingdom
- ARC_/Arthritis Research UK/United Kingdom
- R01 AR069700/AR/NIAMS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- 21156/VAC_/Versus Arthritis/United Kingdom
- 20775/VAC_/Versus Arthritis/United Kingdom
- MC_PC_12009/MRC_/Medical Research Council/United Kingdom
- 20050/VAC_/Versus Arthritis/United Kingdom
- 203151/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- R35 DE027550/DE/NIDCR NIH HHS/United States
- 19429/VAC_/Versus Arthritis/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

