Pancreatic β-cells respond to fuel pressure with an early metabolic switch
- PMID: 32963286
- PMCID: PMC7508987
- DOI: 10.1038/s41598-020-72348-1
Pancreatic β-cells respond to fuel pressure with an early metabolic switch
Abstract
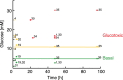
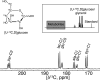
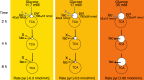
Pancreatic β-cells become irreversibly damaged by long-term exposure to excessive glucose concentrations and lose their ability to carry out glucose stimulated insulin secretion (GSIS) upon damage. The β-cells are not able to control glucose uptake and they are therefore left vulnerable for endogenous toxicity from metabolites produced in excess amounts upon increased glucose availability. In order to handle excess fuel, the β-cells possess specific metabolic pathways, but little is known about these pathways. We present a study of β-cell metabolism under increased fuel pressure using a stable isotope resolved NMR approach to investigate early metabolic events leading up to β-cell dysfunction. The approach is based on a recently described combination of 13C metabolomics combined with signal enhanced NMR via dissolution dynamic nuclear polarization (dDNP). Glucose-responsive INS-1 β-cells were incubated with increasing concentrations of [U-13C] glucose under conditions where GSIS was not affected (2-8 h). We find that pyruvate and DHAP were the metabolites that responded most strongly to increasing fuel pressure. The two major divergence pathways for fuel excess, the glycerolipid/fatty acid metabolism and the polyol pathway, were found not only to operate at unchanged rate but also with similar quantity.
Conflict of interest statement
The authors declare no competing interests.
Figures


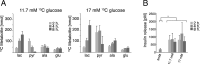


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

