Dose-dense paclitaxel plus carboplatin vs. epirubicin and cyclophosphamide with paclitaxel as adjuvant chemotherapy for high-risk triple-negative breast cancer
- PMID: 32963461
- PMCID: PMC7491545
- DOI: 10.21147/j.issn.1000-9604.2020.04.06
Dose-dense paclitaxel plus carboplatin vs. epirubicin and cyclophosphamide with paclitaxel as adjuvant chemotherapy for high-risk triple-negative breast cancer
Abstract
Objective: The objective of this open-label, randomized study was to compare dose-dense paclitaxel plus carboplatin (PCdd) with dose-dense epirubicin and cyclophosphamide followed by paclitaxel (ECdd-P) as an adjuvant chemotherapy for early triple-negative breast cancer (TNBC).
Methods: We included Chinese patients with high recurrence risk TNBC who underwent primary breast cancer surgery. They were randomly assigned to receive PCdd [paclitaxel 150 mg/m2 on d 1 and carboplatin, the area under the curve, (AUC)=3 on d 2] or ECdd-P (epirubicin 80 mg/m2 divided in 2 d and cyclophosphamide 600 mg/m2 on d 1 for 4 cycles followed by paclitaxel 175 mg/m2 on d 1 for 4 cycles) every 2 weeks with granulocyte colony-stimulating factor (G-CSF) support. The primary endpoint was 3-year disease-free survival (DFS); the secondary endpoints were overall survival (OS) and safety.
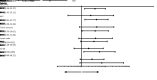
Results: The intent-to-treat population included 143 patients (70 in the PCdd arm and 73 in the ECdd-P arm). Compared with the ECdd-P arm, the PCdd arm had significantly higher 3-year DFS [93.9% vs. 79.1%; hazard ratio (HR)=0.310; 95% confidence interval (95% CI), 0.137-0.704; log-rank, P=0.005] and OS (98.5% vs. 92.9%; HR=0.142; 95% CI, 0.060-0.825; log-rank, P=0.028). Worse neutropenia (grade 3/4) was found in the ECdd-P than the PCdd arm (47.9% vs. 21.4%, P=0.001).
Conclusions: PCdd was superior to ECdd-P as an adjuvant chemotherapy for early TNBC with respect to improving the 3-year DFS and OS. PCdd also yielded lower hematological toxicity. Thus, PCdd might be a preferred regimen for early TNBC patients with a high recurrence risk.
Keywords: Triple-negative breast cancer; carboplatin; dose-dense adjuvant chemotherapy; paclitaxel.
Copyright © 2020 Chinese Journal of Cancer Research. All rights reserved.
Figures



References
-
- Hammond ME, Hayes DF, Dowsett M, et al American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95. doi: 10.1200/JCO.2009.25.6529. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
