MFG-E8 accelerates wound healing in diabetes by regulating "NLRP3 inflammasome-neutrophil extracellular traps" axis
- PMID: 32963812
- PMCID: PMC7484765
- DOI: 10.1038/s41420-020-00318-7
MFG-E8 accelerates wound healing in diabetes by regulating "NLRP3 inflammasome-neutrophil extracellular traps" axis
Abstract
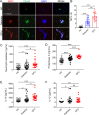
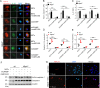
Sustained activation of NLRP3 inflammasome and release of neutrophil extracellular traps (NETs) impair wound healing of diabetic foot ulcers (DFUs). Our previous study reported that milk fat globule epidermal growth factor VIII (MFG-E8) attenuates tissue damage in systemic lupus erythematosus. However, the functional effect of MFG-E8 on "NLRP3 inflammasome-NETs" inflammatory loop in wound healing of diabetes is not completely elucidated. In this study, neutrophils from DFU patients are susceptible to undergo NETosis, releasing more NETs. The circulating levels of NET components neutrophil elastase and proteinase 3 and inflammatory cytokines IL-1β and IL-18 were significantly elevated in DFU patients compared with healthy controls or diabetic patients, in spite of higher levels of MFG-E8 in DFU patients. In Mfge8-/- diabetic mice, skin wound displayed exaggerated inflammatory response, including leukocyte infiltration, excessive activation of NLRP3 inflammasome (release of higher IL-1β, IL-18, and TNF-α), largely lodged NETs, resulting in poor angiogenesis and wound closure. When stimulated with high-dose glucose or IL-18, MFG-E8-deficient neutrophils release more NETs than WT neutrophils. After administration of recombinant MFG-E8, IL-18-primed NETosis of WT or Mfge8-/- neutrophils was significantly inhibited. Furthermore, NET and mCRAMP (component of NETs, the murine equivalent of cathelicidin LL-37 in human)-mediated activation of NLRP3 inflammasome and production of IL-1β/IL-18 were significantly elevated in Mfge8-/- macrophages compared with WT macrophages, which were also significantly dampened by the administration of rmMFG-E8. Therefore, our study demonstrated that as inhibitor of the "NLRP3 inflammasome-NETs" inflammatory loop, exogenous rMFG-E8 improves angiogenesis and accelerates wound healing, highlighting possible therapeutic potential for DFUs.
Keywords: Cell death and immune response; Diabetes complications; Inflammasome.
© The Author(s) 2020.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

