Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer
- PMID: 32963948
- PMCID: PMC7488491
- DOI: 10.1016/j.apsb.2020.02.002
Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer
Abstract
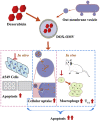
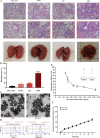
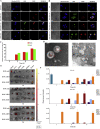
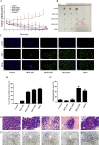
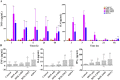
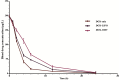
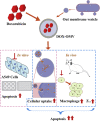
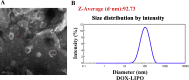
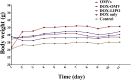
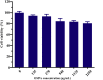
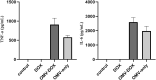
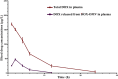
More efficient drug delivery system and formulation with less adverse effects are needed for the clinical application of broad-spectrum antineoplastic agent doxorubicin (DOX). Here we obtained outer-membrane vesicles (OMVs), a nano-sized proteoliposomes naturally released by Gram-negative bacteria, from attenuated Klebsiella pneumonia and prepared doxorubicin-loaded O0MVs (DOX-OMV). Confocal microscopy and in vivo distribution study observed that DOX encapsulated in OMVs was efficiently transported into NSCLC A549 cells. DOX-OMV resulted in intensive cytotoxic effects and cell apoptosis in vitro as evident from MTT assay, Western blotting and flow cytometry due to the rapid cellular uptake of DOX. In A549 tumor-bearing BALB/c nude mice, DOX-OMV presented a substantial tumor growth inhibition with favorable tolerability and pharmacokinetic profile, and TUNEL assay and H&E staining displayed extensive apoptotic cells and necrosis in tumor tissues. More importantly, OMVs' appropriate immunogenicity enabled the recruitment of macrophages in tumor microenvironment which might synergize with their cargo DOX in vivo. Our results suggest that OMVs can not only function as biological nanocarriers for chemotherapeutic agents but also elicit suitable immune responses, thus having a great potential for the tumor chemoimmunotherapy.
Keywords: Anti-tumor efficacy; Bacterial outer-membrane vesicles; Chemoimmunotherapy; Doxorubicin; Non-small-cell lung cancer.
© 2020 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures


References
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca - Cancer J Clin. 2018;68:394–424. - PubMed
-
- Tacar O., Sriamornsak P., Dass C.R. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65:157–170. - PubMed
-
- O’Brien M.E., Wigler N., Inbar M., Rosso R., Grischke E., Santoro A., Catane R.D., Tomczak P., Orlandi F., Mellars L., Alland L., TendlerM C., O'Brien E., Wigler N., Inbar M., Rosso R., Grischke E., Santoro A., Catane R., Kieback D.G., Tomczak P., Ackland S.P., Orlandi F., Mellars L., Alland L., Tendler C. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX™/Doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–449. - PubMed
-
- Barenholz Y. Doxil® — the first FDA-approved nano-drug: lessons learned. J Contr Release. 2012;160:117–134. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

