The Effect of Sample Site, Illness Duration, and the Presence of Pneumonia on the Detection of SARS-CoV-2 by Real-time Reverse Transcription PCR
- PMID: 32964061
- PMCID: PMC7454916
- DOI: 10.1093/ofid/ofaa335
The Effect of Sample Site, Illness Duration, and the Presence of Pneumonia on the Detection of SARS-CoV-2 by Real-time Reverse Transcription PCR
Abstract
Background: The performance of real-time reverse transcription polymerase chain reaction (rRT-PCR) for SARS-CoV-2 varies with sampling site(s), illness stage, and infection site.
Methods: Unilateral nasopharyngeal, nasal midturbinate, throat swabs, and saliva were simultaneously sampled for SARS-CoV-2 rRT-PCR from suspected or confirmed cases of COVID-19. True positives were defined as patients with at least 1 SARS-CoV-2 detected by rRT-PCR from any site on the evaluation day or at any time point thereafter, until discharge. Diagnostic performance was assessed and extrapolated for site combinations.
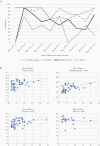
Results: We evaluated 105 patients; 73 had active SARS-CoV-2 infection. Overall, nasopharyngeal specimens had the highest clinical sensitivity at 85%, followed by throat, 80%, midturbinate, 62%, and saliva, 38%-52%. Clinical sensitivity for nasopharyngeal, throat, midturbinate, and saliva was 95%, 88%, 72%, and 44%-56%, respectively, if taken ≤7 days from onset of illness, and 70%, 67%, 47%, 28%-44% if >7 days of illness. Comparing patients with upper respiratory tract infection (URTI) vs pneumonia, clinical sensitivity for nasopharyngeal, throat, midturbinate, and saliva was 92% vs 70%, 88% vs 61%, 70% vs 44%, 43%-54% vs 26%-45%, respectively. A combination of nasopharyngeal plus throat or midturbinate plus throat specimen afforded overall clinical sensitivities of 89%-92%; this rose to 96% for persons with URTI and 98% for persons ≤7 days from illness onset.
Conclusions: Nasopharyngeal specimens, followed by throat specimens, offer the highest clinical sensitivity for COVID-19 diagnosis in early illness. Clinical sensitivity improves and is similar when either midturbinate or nasopharyngeal specimens are combined with throat specimens. Upper respiratory specimens perform poorly if taken after the first week of illness or if there is pneumonia.
Keywords: SARS-CoV-2; illness duration; pneumonia; rRT-PCR; sample site.
© The Author(s) 2020. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures
References
-
- Ginocchio CC, McAdam AJ. Current best practices for respiratory virus testing. J Clin Microbiol 2011; 49:44–8.
-
- Frazee BW, Rodríguez-Hoces de la Guardia A, Alter H, et al. . Accuracy and discomfort of different types of intranasal specimen collection methods for molecular influenza testing in emergency department patients. Ann Emerg Med 2018; 71:509–17.e1. - PubMed
-
- He X, Lau EHY, Wu P, et al. . Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020; 26:672–5. - PubMed
-
- Wölfel R, Corman VM, Guggemos W, et al. . Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581:465–9. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous