Feasibility and first reports of the MATCH-R repeated biopsy trial at Gustave Roussy
- PMID: 32964129
- PMCID: PMC7478969
- DOI: 10.1038/s41698-020-00130-7
Feasibility and first reports of the MATCH-R repeated biopsy trial at Gustave Roussy
Abstract
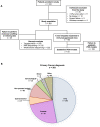
Unravelling the biological processes driving tumour resistance is necessary to support the development of innovative treatment strategies. We report the design and feasibility of the MATCH-R prospective trial led by Gustave Roussy with the primary objective of characterizing the molecular mechanisms of resistance to cancer treatments. The primary clinical endpoints consist of analyzing the type and frequency of molecular alterations in resistant tumours and compare these to samples prior to treatment. Patients experiencing disease progression after an initial partial response or stable disease for at least 24 weeks underwent a tumour biopsy guided by CT or ultrasound. Molecular profiling of tumours was performed using whole exome sequencing, RNA sequencing and panel sequencing. At data cut-off for feasibility analysis, out of 333 inclusions, tumour biopsies were obtained in 303 cases (91%). From these biopsies, 278 (83%) had sufficient quality for analysis by high-throughput next generation sequencing (NGS). All 278 samples underwent targeted NGS, 215 (70.9%) RNA sequencing and 222 (73.2%) whole exome sequencing. In total, 163 tumours were implanted in NOD scid gamma (NSG) or nude mice and 54 patient-derived xenograft (PDX) models were established, with a success rate of 33%. Adverse events secondary to invasive tumour sampling occurred in 24 patients (7.6%). Study recruitment is still ongoing. Systematic molecular profiling of tumours and the development of patient-derived models of acquired resistance to targeted agents and immunotherapy is feasible and can drive the selection of the next therapeutic strategy.
Keywords: Biomarkers; Cancer genetics.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsG.R. Received travel accommodations, congress registration expenses from Pfizer. Consulting, advisory role: Pfizer, Amgen and Roche. Y.L. Received grants, personal fees and non-financial support from JANSSEN; personal fees and non-financial support from ASTELLAS, grants and personal fees from SANOFI, personal fees and non-financial support from ROCHE, personal fees and non-financial support from ASTRA ZENECA, grants, personal fees and non-financial support from MSD, personal fees and non-financial support from BMS, personal fees from CLOVIS, personal fees and non-financial support from SEATTLE GENETICS, personal fees from INCYTE, personal fees from PFIZER, outside the submitted work; In addition, Dr. LORIOT has a patent US: 62/455211 EUROPE:17209098.7 pending. A.G. Received travel accommodations, congress registration expenses from Boehringer Ingelheim, Novartis, Pfizer, and Roche. Consultant/Expert role for Novartis. Principal/sub-Investigator of Clinical Trials for Aduro Biotech, Agios Pharmaceuticals, Amgen, Argen-X Bvba, Arno Therapeutics, Astex Pharmaceuticals, Astra Zeneca, Aveo, Bayer Healthcare Ag, Bbb Technologies Bv, Beigene, Bioalliance Pharma, Biontech Ag, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Ca, Celgene Corporation, Chugai Pharmaceutical Co., Clovis Oncology, Daiichi Sankyo, Debiopharm S.A., Eisai, Exelixis, Forma, Gamamabs, Genentech, Inc., Gilead Sciences, Inc., GlaxoSmithKline, Glenmark Pharmaceuticals, H3 Biomedicine, Inc., Hoffmann La Roche Ag, Incyte Corporation, Innate Pharma, Iris Servier, Janssen, Kura Oncology, Kyowa Kirin Pharm, Lilly, Loxo Oncology, Lytix Biopharma As, Medimmune, Menarini Ricerche, Merck Sharp & Dohme Chibret, Merrimack Pharmaceuticals, Merus, Millennium Pharmaceuticals, Nanobiotix, Nektar Therapeutics, Novartis Pharma, Octimet Oncology Nv, Oncoethix, Oncomed, Oncopeptides, Onyx Therapeutics, Orion Pharma, Oryzon Genomics, Pfizer, Pharma Mar, Pierre Fabre, Rigontec Gmbh, Roche, Sanofi Aventis, Sierra Oncology, Taiho Pharma, Tesaro, Inc., Tioma Therapeutics, Inc., Xencor. Research Grants from AstraZeneca, BMS, Boehringer Ingelheim, Janssen Cilag, Merck, Novartis, Pfizer, Roche, Sanofi. Non-financial support (drug supplied) from AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Johnson & Johnson, Lilly, Medimmune, Merck, NH TherAGuiX, Pfizer, Roche. A.H. Consultant/Advisory role for Amgen, Spectrum Pharmaceuticals, Lilly. Invitations to national or international congresses from Servier, Amgen, Lilly Courses, trainings for Bayer. Principal/sub-Investigator of Clinical Trials for AbbVie, Agios Pharmaceuticals, Amgen, Argen-X Bvba, Arno Therapeutics, Astex Pharmaceuticals, Astra Zeneca, Aveo, Bayer Healthcare Ag, Bbb Technologies Bv, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Celgene Corporation, Chugai Pharmaceutical Co., Clovis Oncology, Daiichi Sankyo, Debiopharm S.A., Eisai, Eli Lilly, Exelixis, Forma, Gamamabs, Genentech, Inc., GlaxoSmithKline, H3 Biomedicine, Inc., Hoffmann La Roche Ag, Innate Pharma, Iris Servier, Janssen Cilag, Kyowa Kirin Pharm. Dev., Inc., Loxo Oncology, Lytix Biopharma As, Medimmune, Menarini Ricerche, Merck Sharp & Dohme Chibret, Merrimack Pharmaceuticals, Merus, Millennium Pharmaceuticals, Nanobiotix, Nektar Therapeutics, Novartis Pharma, Octimet Oncology Nv, Oncoethix, Onyx Therapeutics, Orion Pharma, Oryzon Genomics, Pfizer, Pharma Mar, Pierre Fabre, Roche, Sanofi Aventis, Taiho Pharma, Tesaro, Inc., Xencor. D.P. Consulting, advisory role or lectures: AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Merck, MedImmune, Novartis, Pfizer, prIME Oncology, Peer CME, Roche. Honoraria: AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Merck, Novartis, Pfizer, prIME Oncology, Peer CME, Roche. Clinical trials research as principal or co-investigator (Institutional financial interests): AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, Pfizer, Roche, Medimmune, Sanofi Aventis, Taiho Pharma, Novocure, and Daiichi Sanky. Travel, Accommodations, Expenses: AstraZeneca, Roche, Novartis, prIME Oncology, Pfizer. L.M: Consulting, advisory role: Roche Diagnostics. Lectures and educational activities: Bristol Myers Squibb, Tecnofarma, Roche, AstraZeneca. Travel, Accommodations, Expenses: Chugai. P.L. Travel accomodations: Astellas-Pharma, Astra Zeneca, Ipsen, Janssen Oncology. T.D.B. proctor for Cook medical, speaker and expert for GE Healthcare. O.D. is an employee of XenTech. E.A. Consulting or Advisory Role: Merck Sharp & Dohme, GlaxoSmithKline, Celgene Research, MedImmune. Travel, Accommodations, Expenses: AbbVie, Roche, Sanofi, Pfizer, MedImmune. Principal/sub-Investigator of Clinical Trials (Inst.) for AbbVie, Aduro, Agios, Amgen, Argen-x, Astex, AstraZeneca, Aveo pharmaceuticals, Bayer, Beigene, Blueprint, BMS, Boeringer Ingelheim, Celgene, Chugai, Clovis, Daiichi Sankyo, Debiopharm, Eisai, Eos, Exelixis, Forma, Gamamabs, Genentech, Gortec, GSK, H3 biomedecine, Incyte, Innate Pharma, Janssen, Kura Oncology, Kyowa, Lilly, Loxo, Lysarc, Lytix Biopharma, Medimmune, Menarini, Merus, MSD, Nanobiotix, Nektar Therapeutics, Novartis, Octimet, Oncoethix, Oncopeptides AB, Orion, Pfizer, Pharmamar, Pierre Fabre, Roche, Sanofi, Servier, Sierra Oncology, Taiho, Takeda, Tesaro, Xencor. A.E. Honoraria over last 5 years for any speaker, consultancy or advisory role from: Actelion, Agenus, Bayer, BMS, CellDex, Ellipses, Gilead, GSK, HalioDX, Incyte, IO Biotech, ISA pharmaceuticals, MedImmune, Merck GmbH, MSD, Nektar, Novartis, Pfizer, Polynoma, Regeneron, RiverDx, Sanofi, Sellas, SkylineDx. E.A. Consulting or Advisory Role: Merck Sharp & Dohme, GlaxoSmithKline, Celgene Research, MedImmune. Travel, Accommodations, Expenses: AbbVie, Roche, Sanofi, Pfizer, MedImmune. Principal/sub-Investigator of Clinical Trials (Inst.) for AbbVie, Aduro, Agios, Amgen, Argen-x, Astex, AstraZeneca, Aveo pharmaceuticals, Bayer, Beigene, Blueprint, BMS, Boeringer Ingelheim, Celgene, Chugai, Clovis, Daiichi Sankyo, Debiopharm, Eisai, Eos, Exelixis, Forma, Gamamabs, Genentech, Gortec, GSK, H3 biomedecine, Incyte, Innate Pharma, Janssen, Kura Oncology, Kyowa, Lilly, Loxo, Lysarc, Lytix Biopharma, Medimmune, Menarini, Merus, MSD, Nanobiotix, Nektar Therapeutics, Novartis, Octimet, Oncoethix, Oncopeptides AB, Orion, Pfizer, Pharmamar, Pierre Fabre, Roche, Sanofi, Servier, Sierra Oncology, Taiho, Takeda, Tesaro, Xencor. F.A. travel/accommodation/expenses from AstraZeneca, GlaxoSmithKline, Novartis, and Roche, and his institution has received research funding from AstraZeneca, Lilly, Novartis, Pfizer, and Roche. C.M. Consultant/Advisory fees from Amgen, Astellas, Astra Zeneca, Bayer, BeiGene, BMS, Celgene, Debiopharm, Genentech, Ipsen, Janssen, Lilly, MedImmune, MSD, Novartis, Pfizer, Roche, Sanofi, Orion. Principal/sub-Investigator of Clinical Trials for AbbVie, Aduro, Agios, Amgen, Argen-x, Astex, AstraZeneca, Aveo pharmaceuticals, Bayer, Beigene, Blueprint, BMS, Boeringer Ingelheim, Celgene, Chugai, Clovis, Daiichi Sankyo, Debiopharm, Eisai, Eos, Exelixis, Forma, Gamamabs, Genentech, Gortec, GSK, H3 biomedecine, Incyte, InnatePharma, Janssen, Kura Oncology, Kyowa, Lilly, Loxo, Lysarc, Lytix Biopharma, Medimmune, Menarini, Merus, MSD, Nanobiotix, Nektar Therapeutics, Novartis, Octimet, Oncoethix, Oncopeptides AB, Orion, Pfizer, Pharmamar, Pierre Fabre, Roche, Sanofi, Servier, Sierra Oncology, Taiho, Takeda, Tesaro, Xencor. J.C.S. Over the last 5 years, consultancy fees from AstraZeneca, Astex, Clovis, GSK, GamaMabs, Lilly, MSD, Mission Therapeutics, Merus, Pfizer, PharmaMar, Pierre Fabre, Roche/Genentech, Sanofi, Servier, Symphogen, and Takeda. Dr Soria has been a full-time employee of AstraZeneca between September 2017 and January 2020. He is a shareholder of AstraZeneca and Gritstone. B.B. Received institutional grants for clinical and translational research from AstraZeneca, Boehringer ingelheim, Bristol Myers Squibb (BMS), Inivata, Lilly, Loxo, OncoMed, Onxeo, Pfizer, Roche-Genentech, Sanofi Aventis, Servier, and OSE Pharma. All other authors declare no competing interests.
Figures


References
-
- Tang, J., Pearce, L., O’Donnell-Tormey, J. & Hubbard-Lucey, V. M. Trends in the global immuno-oncology landscape. Nat. Rev. Drug Discov. 17, 783–784 (2018). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

