Assessment of the clinical utility of pharmacogenetic guidance in a comprehensive medication management service
- PMID: 32964197
- PMCID: PMC7505210
- DOI: 10.1002/jac5.1250
Assessment of the clinical utility of pharmacogenetic guidance in a comprehensive medication management service
Abstract
Introduction: Pharmacists are poised to be the health care professionals best suited to provide medication-related consults and services based on a patient's genetics. Despite its potential benefits, the implementation of pharmacogenetic (PGx) testing into primary clinical settings has been slow among medically underserved populations. To our knowledge, this is the first time that PGx-driven recommendations have been incorporated into a Comprehensive Medication Management (CMM) service in a Hispanic population.
Objectives: The aim of this study is to evaluate the clinical utility of adding PGx guidance into pharmacist-driven CMM.
Methods: This is a pre- and post-interventional design study. Patients were recruited from a psychologist's clinic. A total of 24 patients had a face-to-face interview with a pharmacist to complete a CMM, Personal Medication Record, and Medication-Related Action Plan (MAP) blind to PGx findings. Collected buccal DNA samples were genotyped using drug-metabolizing enzymes and transporters (DMET) Plus Array.
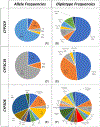
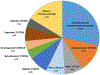
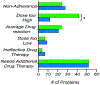
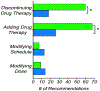
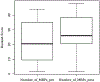
Results: The pharmacist generated new MAPs for each patient based on PGx results. Genetic variants that could potentially affect the safety and effectiveness of at least one drug in the pharmacotherapy were identified in 96% of patients, for whom the pharmacist changed the initial recommendations. Polymorphisms in genes encoding for isoenzymes CYP2D6, CYP2C19, and CYP2C9 were identified in 83%, 52%, and 41% of patients, respectively. Pharmacists performing CMM identified 22 additional medication problems after PGx determinations. Moreover, they agreed with the clinical utility of PGx in the studied sample based on perceived value of adding PGx to traditional CMM and its utility in the decision-making process of pharmacists.
Conclusions: The study confirmed the critical role to be played by pharmacists in facilitating the clinical usage of relevant genetic information to optimize drug therapy decisions as well as their involvement on many levels of these multidisciplinary implementation efforts, including championing and leading PGx-guided CMM services.
Keywords: clinical decision support; cytochrome P450; pharmacogenetics.
Conflict of interest statement
CONFLICT OF INTEREST Gualberto Ruaño is a founder and medical director of the Genomas, Inc. The authors have no further conflict of interest to declare.
Figures
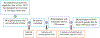
Similar articles
-
Clinical Utility of Pharmacogenetic Testing and a Clinical Decision Support Tool to Enhance the Identification of Drug Therapy Problems Through Medication Therapy Management in Polypharmacy Patients.J Manag Care Spec Pharm. 2018 Dec;24(12):1250-1259. doi: 10.18553/jmcp.2018.24.12.1250. J Manag Care Spec Pharm. 2018. PMID: 30479202 Free PMC article. Clinical Trial.
-
Pharmacogenetic testing and counselling in the community pharmacy: mixed-methods study of a new pharmacist-led service.Int J Clin Pharm. 2023 Dec;45(6):1378-1386. doi: 10.1007/s11096-023-01596-8. Epub 2023 Jun 20. Int J Clin Pharm. 2023. PMID: 37338707 Free PMC article.
-
Nine-gene pharmacogenomics profile service: The Mayo Clinic experience.Pharmacogenomics J. 2022 Feb;22(1):69-74. doi: 10.1038/s41397-021-00258-0. Epub 2021 Oct 20. Pharmacogenomics J. 2022. PMID: 34671112
-
Making the economic value proposition for pharmacist comprehensive medication management (CMM) in primary care: A conceptual framework.Res Social Adm Pharm. 2020 Oct;16(10):1416-1421. doi: 10.1016/j.sapharm.2020.01.001. Epub 2020 Jan 2. Res Social Adm Pharm. 2020. PMID: 31918964 Review.
-
Applications for pharmacogenomics in pharmacy practice: A scoping review.Res Social Adm Pharm. 2022 Jul;18(7):3094-3118. doi: 10.1016/j.sapharm.2021.08.009. Epub 2021 Aug 20. Res Social Adm Pharm. 2022. PMID: 34474980
Cited by
-
The Critical Role of Pharmacists in the Clinical Delivery of Pharmacogenetics in the U.S.Pharmacy (Basel). 2023 Sep 10;11(5):144. doi: 10.3390/pharmacy11050144. Pharmacy (Basel). 2023. PMID: 37736916 Free PMC article. Review.
-
The feasibility and potential of pharmacogenetics to reduce adverse drug events in nursing home residents.J Am Geriatr Soc. 2022 May;70(5):1573-1578. doi: 10.1111/jgs.17679. Epub 2022 Feb 14. J Am Geriatr Soc. 2022. PMID: 35157308 Free PMC article. No abstract available.
-
Prescription Advice Based on Data of Drug-Drug-Gene Interaction of Patients with Polypharmacy.Pharmgenomics Pers Med. 2022 Aug 18;15:765-773. doi: 10.2147/PGPM.S368606. eCollection 2022. Pharmgenomics Pers Med. 2022. PMID: 36004008 Free PMC article.
References
-
- American Pharmacists Association (APhA) Policy. 2018. Annual Meeting & Exposition Leading our Communities in Patient Care: Actions of the 2018 American Pharmacists Association House of Delegates; March 16-19, 2018; Nashville, Tennessee: Available at https://www.pharmacist.com/sites/default/files/files/2018%20Report%20of%.... [Accessed on March 22, 2020].
-
- Hocum BT, White JR Jr, Heck JW, et al. Cytochrome P-450 gene and drug interaction analysis in patients referred for pharmacogenetic testing. Am J Health Syst Pharm. 2016;73(2):61–67. - PubMed
-
- American Society of Health-System Pharmacists. ASHP statement on the pharmacist's role in clinical pharmacogenomics. Am J Health Syst Pharm. 2015;72(7):579–581. - PubMed
-
- McInnis T, Webb CE, Strand LM. The patient-centered medical home: Integrating comprehensive medication management to optimize patient outcomes. 2nd ed.Washington, DC: Patient-Centered Primary Care Collaborative, 2012;p. 1–28 Available at https://www.accp.com/docs/positions/misc/CMMResourceGuide.pdf. [Accessed on March 22, 2020].
Grants and funding
LinkOut - more resources
Full Text Sources
