Transfusion reactions in pediatric and adolescent young adult haematology oncology and immune effector cell patients
- PMID: 32964199
- PMCID: PMC7490993
- DOI: 10.1016/j.eclinm.2020.100514
Transfusion reactions in pediatric and adolescent young adult haematology oncology and immune effector cell patients
Abstract
Background: Active surveillance for transfusion reactions is critically important among pediatric patients undergoing chemotherapy. Among pediatric-adolescent-young-adult (AYA) hematology/oncology patients, who have been typically excluded from transfusion reaction studies, this profile remains poorly characterized.
Methods: We assessed the incidence and clinical characteristics of transfusion reactions (n = 3246 transfusions) in this population (n = 201 patients) at our center.
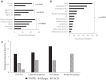
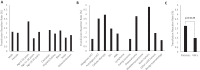
Findings: The incidence of adjudicated transfusion reactions was 2·04%. The incidence was higher for platelet (2·78%) compared to packed red blood cell transfusions (1·49%) (p = 0·0149). The majority (61·4%) of all reactions were classified as febrile non-haemolytic transfusion, while 35·7% were considered allergic, and 2·9% were classified as transfusion-associated circulatory overload. The incidence of transfusion reactions in patients who were pre-medicated was higher (2·51%) than in patients who were not (1·52%) (p = 0·0406). Sub-set analysis revealed a 3·95% incidence of adjudicated transfusion reactions among recipients of immune effector cells (IECs) (n = 3), all of which occurred during the potential window for cytokine release syndrome; two-thirds of these reactions were severe/potentially life-threatening.
Interpretation: The incidence of transfusion reactions among pediatric-AYA hematology/oncology patients may be lower than the general pediatric population. Patients with a prior history of transfusion reactions and those receiving platelet transfusions may be at higher risk for reaction. From our limited sample, IEC recipients may be at risk for severe transfusion reactions. Large multi-center prospective studies are needed to characterize transfusion reactions in this population. Appropriate characterization of reactions in this population may inform risk stratification and mitigate missed opportunities for prompt recognition and appropriate management.
Funding: None.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Transfusion reactions in pediatric compared with adult patients: a look at rate, reaction type, and associated products.Transfusion. 2015 Mar;55(3):563-70. doi: 10.1111/trf.12827. Epub 2014 Aug 22. Transfusion. 2015. PMID: 25145580
-
Analysis of pediatric adverse reactions to transfusions.Transfusion. 2018 Jan;58(1):60-69. doi: 10.1111/trf.14359. Epub 2017 Sep 25. Transfusion. 2018. PMID: 28948619
-
Contribution of donor- and recipient-associated factors to allergic transfusion reactions to platelets.Transfusion. 2021 Mar;61(3):744-753. doi: 10.1111/trf.16221. Epub 2020 Dec 13. Transfusion. 2021. PMID: 33314235
-
Pediatric red cell and platelet transfusions.Transfus Apher Sci. 2018 Jun;57(3):358-362. doi: 10.1016/j.transci.2018.05.019. Epub 2018 May 16. Transfus Apher Sci. 2018. PMID: 29804934 Review.
-
Clinical significance of RBC alloantibodies and autoantibodies in sickle cell patients who received transfusions.Transfusion. 2002 Jan;42(1):37-43. doi: 10.1046/j.1537-2995.2002.00007.x. Transfusion. 2002. PMID: 11896310 Review.
Cited by
-
Effect of donor and red blood cells concentrate characteristics on recipient hemoglobin increment following red blood cells transfusion in pediatric patients.Pak J Med Sci. 2022 Jul-Aug;38(6):1420-1425. doi: 10.12669/pjms.38.6.5739. Pak J Med Sci. 2022. PMID: 35991248 Free PMC article.
-
Incidence of acute transfusion reactions and associated factors among adult blood-transfused patients at Jimma University Medical Center, southwest Ethiopia: A cross-sectional study.Medicine (Baltimore). 2024 Aug 9;103(32):e39137. doi: 10.1097/MD.0000000000039137. Medicine (Baltimore). 2024. PMID: 39121245 Free PMC article.
-
Diagnosis, grading and management of toxicities from immunotherapies in children, adolescents and young adults with cancer.Nat Rev Clin Oncol. 2021 Jul;18(7):435-453. doi: 10.1038/s41571-021-00474-4. Epub 2021 Feb 19. Nat Rev Clin Oncol. 2021. PMID: 33608690 Free PMC article. Review.
References
-
- Lieberman L., Liu Y., Portwine C., Barty R.L., Heddle N.M. An epidemiologic cohort study reviewing the practice of blood product transfusions among a population of pediatric oncology patients. Transfusion. 2014;54(10 Pt 2):2736–2744. - PubMed
-
- Delaney M., Wendel S., Bercovitz R.S. Transfusion reactions: prevention, diagnosis, and treatment. Lancet. 2016;388(10061):2825–2836. - PubMed
-
- U.S. Centers for Disease Control and Prevention. The National Healthcare Safety Network (NHSN) Manual: biovigilance Component v2.5. Atlanta GDoHQP, National Center for Emerging and Zoonotic Infectious Diseases. Available at: http://www.cdc.gov/nhsn/PDFs/Biovigilance/BV-HV-protocol-current.pdf. Accessed 3 June 2020.
-
- Oakley F.D., Woods M., Arnold S., Young P.P. Transfusion reactions in pediatric compared with adult patients: a look at rate, reaction type, and associated products. Transfusion. 2015;55(3):563–570. - PubMed
LinkOut - more resources
Full Text Sources

