Safety of Ustekinumab in Inflammatory Bowel Disease: Pooled Safety Analysis of Results from Phase 2/3 Studies
- PMID: 32964215
- PMCID: PMC8205635
- DOI: 10.1093/ibd/izaa236
Safety of Ustekinumab in Inflammatory Bowel Disease: Pooled Safety Analysis of Results from Phase 2/3 Studies
Erratum in
-
Corrigendum to: Safety of Ustekinumab in Inflammatory Bowel Disease: Pooled Safety Analysis of Results from Phase 2/3 Studies.Inflamm Bowel Dis. 2021 Jun 15;27(7):1175. doi: 10.1093/ibd/izab014. Inflamm Bowel Dis. 2021. PMID: 33511983 Free PMC article. No abstract available.
Abstract
Background: Ustekinumab is currently approved globally in Crohn's disease (CD) and psoriatic diseases. Recent phase 3 data demonstrate safety/efficacy in ulcerative colitis (UC). Crohn's disease and UC phase 3 programs had similar study designs, facilitating integrated safety analyses.
Methods: Data from 6 ustekinumab phase 2/3 CD and UC studies were pooled, and safety was evaluated through 1 year. Patients received 1 placebo or ustekinumab (generally 130 mg or ~6 mg/kg) intravenous induction, then subcutaneous (90 mg) maintenance every 8/12 weeks. Analyses incorporated all patients who received ≥1 ustekinumab dose. Safety outcomes are presented as percentages of patients (induction) and as number of patients with events per 100 patient-years of follow-up (through 1 year). For key safety events, 95% confidence intervals (CIs) are provided, as appropriate. Hazard ratios with 95% CIs from time-to-event analyses for serious adverse events and serious infections were also performed.
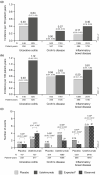
Results: Through 1 year, 2574 patients received ustekinumab (1733 patient-years of follow-up). The number of patients with adverse events per 100 patient-years (placebo 165.99 [95% CI, 155.81-176.67] vs ustekinumab 118.32 [95% CI, 113.25-123.55]), serious AEs (27.50 [95% CI, 23.45-32.04] vs 21.23 [95% CI, 19.12-23.51]), infections (80.31 [95% CI, 73.28-87.84] vs 64.32 [95% CI, 60.60-68.21]), serious infections (5.53 [95% CI, 3.81-7.77] vs 5.02 [95% CI, 4.02-6.19]), and malignancies excluding nonmelanoma skin cancer (0.17 [95% CI, 0.00-0.93] vs 0.40 [95% CI, 0.16-0.83]) were similar between placebo and ustekinumab.
Conclusions: The safety profile of ustekinumab across the pooled inflammatory bowel disease population through 1 year was favorable and generally comparable to placebo. These data are consistent with the established safety profile of ustekinumab across indications.
Clinicaltrials.gov numbers: NCT00265122; NCT00771667; NCT01369329; NCT01369342; NCT01369355; NCT02407236.
Keywords: inflammatory bowel disease; safety; ustekinumab.
© 2020 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Figures
References
-
- Kimball AB, Gordon KB, Fakharzadeh S, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial through up to 3 years. Br J Dermatol. 2012;166:861–872. - PubMed
-
- Kimball AB, Papp KA, Wasfi Y, et al. ; PHOENIX 1 Investigators . Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535–1545. - PubMed
-
- Leonardi CL, Kimball AB, Papp KA, et al. ; PHOENIX 1 study investigators . Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371: 1665–1674. - PubMed
-
- Sandborn WJ, Gasink C, Gao LL, et al. ; CERTIFI Study Group . Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–1528. - PubMed
-
- Feagan BG, Sandborn WJ, Gasink C, et al. ; UNITI–IM-UNITI Study Group . Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960. - PubMed

