Epithelial Cell Biomarkers Are Predictive of Response to Biologic Agents in Crohn's Disease
- PMID: 32964238
- PMCID: PMC8047859
- DOI: 10.1093/ibd/izaa251
Epithelial Cell Biomarkers Are Predictive of Response to Biologic Agents in Crohn's Disease
Abstract
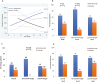
Background: Therapeutic efficacy of biologics has remained at about 50% for 2 decades. In Crohn's disease (CD) patients, we examined the predictive value of an epithelial cell biomarker, ileal microvillar length (MVL), for clinical response to ustekinumab (UST) and vedolizumab (VDZ) and its relationship to another biomarker, intestinal epithelial cell (IEC) pyroptosis, with respect to response to VDZ.
Method: Ileal biopsies from the UNITI-2 randomized controlled trial were analyzed for MVL as a predictor of clinical response to UST. In a 5-center academic retrospective cohort of CD patients, ileal MVL was analyzed to determine its predictive value for response to VDZ. Correlation between ileal MVL and IEC pyroptosis was determined, and the discriminant ability of the combination of 2 biomarkers to VDZ was examined.
Results: Clinical response in UST was significantly higher than placebo (65% vs 39%; P = 0.03), with patients with normal MVL (>1.7 µm) having the greatest therapeutic effect: 85% vs 20% (P = 0.02). For VDZ, clinical response with MVL of 1.35 to 1.55 µm was 82% vs 44% (<1.35 µm) and 40% (>1.55 µm; P = 0.038). There was no correlation between ileal MVL and IEC pyroptosis. The combination criteria of ileal pyroptosis <14 positive cells/1000 IECs or MVL of 1.35 to 1.55 µm could identify 84% of responders and 67% of nonresponders (P = 0.001).
Conclusion: Ileal MVL was predictive of response to UST and VDZ in prospective and retrospective CD cohorts. It was independent of ileal IEC pyroptosis, and combination of the 2 biomarkers enhanced the discriminate ability of responders from nonresponders to VDZ.
Keywords: biomarker; clinical remission; clinical response; endoscopic improvement; epithelial cells.
© 2020 Crohn’s & Colitis Foundation. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



Similar articles
-
Mucosal Biomarker of Innate Immune Activation Predicts Response to Vedolizumab in Crohn's Disease.Inflamm Bowel Dis. 2020 Sep 18;26(10):1554-1561. doi: 10.1093/ibd/izz222. Inflamm Bowel Dis. 2020. PMID: 31553433
-
Real-world clinical outcomes and healthcare costs in patients with Crohn's disease treated with vedolizumab versus ustekinumab in the United States.Curr Med Res Opin. 2024 May;40(5):877-885. doi: 10.1080/03007995.2024.2326585. Epub 2024 Apr 8. Curr Med Res Opin. 2024. PMID: 38586979
-
Efficacy of Ustekinumab and Vedolizumab Among Postoperative Crohn's Disease Patients as Postoperative Prophylaxis and Rescue Therapy: Real-world Data.Inflamm Bowel Dis. 2025 Feb 6;31(2):461-466. doi: 10.1093/ibd/izae137. Inflamm Bowel Dis. 2025. PMID: 38953641
-
Therapeutic Drug Monitoring With Ustekinumab and Vedolizumab in Inflammatory Bowel Disease.Inflamm Bowel Dis. 2018 Sep 15;24(10):2165-2172. doi: 10.1093/ibd/izy134. Inflamm Bowel Dis. 2018. PMID: 29788272 Review.
-
Anti-IL-12/23p40 antibodies for maintenance of remission in Crohn's disease.Cochrane Database Syst Rev. 2019 Dec 12;12(12):CD012804. doi: 10.1002/14651858.CD012804.pub2. Cochrane Database Syst Rev. 2019. PMID: 31828765 Free PMC article.
Cited by
-
Baseline Peripheral Blood Mononuclear Cell Transcriptomics Before Ustekinumab Treatment Is Linked With Crohn's Disease Clinical Response at 1 Year.Clin Transl Gastroenterol. 2023 Dec 1;14(12):e00635. doi: 10.14309/ctg.0000000000000635. Clin Transl Gastroenterol. 2023. PMID: 37655708 Free PMC article.
-
Small intestinal immunopathology and GI-associated antibody formation in hereditary alpha-tryptasemia.J Allergy Clin Immunol. 2021 Sep;148(3):813-821.e7. doi: 10.1016/j.jaci.2021.04.004. Epub 2021 Apr 15. J Allergy Clin Immunol. 2021. PMID: 33865872 Free PMC article.
-
Ustekinumab in the Treatment of Crohn's Disease-A Narrative Review on Clinical Efficacy and Safety Profile.Pharmacy (Basel). 2025 May 21;13(3):73. doi: 10.3390/pharmacy13030073. Pharmacy (Basel). 2025. PMID: 40407511 Free PMC article. Review.
-
A pilot study to identify blood-based markers associated with response to treatment with Vedolizumab in patients with Inflammatory Bowel Disease.medRxiv [Preprint]. 2024 Sep 22:2024.09.19.24314034. doi: 10.1101/2024.09.19.24314034. medRxiv. 2024. Update in: Immunol Cell Biol. 2025 Aug;103(7):648-663. doi: 10.1111/imcb.70039. PMID: 39371119 Free PMC article. Updated. Preprint.
-
Predictive, preventive and personalised approach as a conceptual and technological innovation in primary and secondary care of inflammatory bowel disease benefiting affected individuals and populations.EPMA J. 2024 Feb 9;15(1):111-123. doi: 10.1007/s13167-024-00351-x. eCollection 2024 Mar. EPMA J. 2024. PMID: 38463620 Free PMC article. Review.
References
-
- Targan SR, Hanauer SB, van Deventer SJ, et al. . A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035. - PubMed
-
- Present DH, Rutgeerts P, Targan S, et al. . Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398–1405. - PubMed
-
- Rutgeerts P, Sandborn WJ, Feagan BG, et al. . Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. - PubMed
-
- Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group . Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. - PubMed
-
- Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group . Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

