Epithelial Cell Biomarkers Are Predictive of Response to Biologic Agents in Crohn's Disease
- PMID: 32964238
- PMCID: PMC8047859
- DOI: 10.1093/ibd/izaa251
Epithelial Cell Biomarkers Are Predictive of Response to Biologic Agents in Crohn's Disease
Abstract
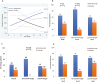
Background: Therapeutic efficacy of biologics has remained at about 50% for 2 decades. In Crohn's disease (CD) patients, we examined the predictive value of an epithelial cell biomarker, ileal microvillar length (MVL), for clinical response to ustekinumab (UST) and vedolizumab (VDZ) and its relationship to another biomarker, intestinal epithelial cell (IEC) pyroptosis, with respect to response to VDZ.
Method: Ileal biopsies from the UNITI-2 randomized controlled trial were analyzed for MVL as a predictor of clinical response to UST. In a 5-center academic retrospective cohort of CD patients, ileal MVL was analyzed to determine its predictive value for response to VDZ. Correlation between ileal MVL and IEC pyroptosis was determined, and the discriminant ability of the combination of 2 biomarkers to VDZ was examined.
Results: Clinical response in UST was significantly higher than placebo (65% vs 39%; P = 0.03), with patients with normal MVL (>1.7 µm) having the greatest therapeutic effect: 85% vs 20% (P = 0.02). For VDZ, clinical response with MVL of 1.35 to 1.55 µm was 82% vs 44% (<1.35 µm) and 40% (>1.55 µm; P = 0.038). There was no correlation between ileal MVL and IEC pyroptosis. The combination criteria of ileal pyroptosis <14 positive cells/1000 IECs or MVL of 1.35 to 1.55 µm could identify 84% of responders and 67% of nonresponders (P = 0.001).
Conclusion: Ileal MVL was predictive of response to UST and VDZ in prospective and retrospective CD cohorts. It was independent of ileal IEC pyroptosis, and combination of the 2 biomarkers enhanced the discriminate ability of responders from nonresponders to VDZ.
Keywords: biomarker; clinical remission; clinical response; endoscopic improvement; epithelial cells.
© 2020 Crohn’s & Colitis Foundation. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035. - PubMed
-
- Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398–1405. - PubMed
-
- Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. - PubMed
-
- Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group . Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. - PubMed
-
- Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group . Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

