Fibulin-3 knockout mice demonstrate corneal dysfunction but maintain normal retinal integrity
- PMID: 32964303
- PMCID: PMC7606609
- DOI: 10.1007/s00109-020-01974-z
Fibulin-3 knockout mice demonstrate corneal dysfunction but maintain normal retinal integrity
Abstract
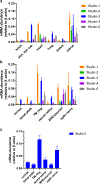
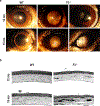
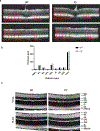
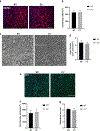
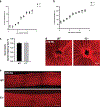
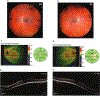
Fibulin-3 (F3) is an extracellular matrix glycoprotein found in basement membranes across the body. An autosomal dominant R345W mutation in F3 causes a macular dystrophy resembling dry age-related macular degeneration (AMD), whereas genetic removal of wild-type (WT) F3 protects mice from sub-retinal pigment epithelium (RPE) deposit formation. These observations suggest that F3 is a protein which can regulate pathogenic sub-RPE deposit formation in the eye. Yet the precise role of WT F3 within the eye is still largely unknown. We found that F3 is expressed throughout the mouse eye (cornea, trabecular meshwork (TM) ring, neural retina, RPE/choroid, and optic nerve). We next performed a thorough structural and functional characterization of each of these tissues in WT and homozygous (F3-/-) knockout mice. The corneal stroma in F3-/- mice progressively thins beginning at 2 months, and the development of corneal opacity and vascularization starts at 9 months, which worsens with age. However, in all other tissues (TM, neural retina, RPE, and optic nerve), gross structural anatomy and functionality were similar across WT and F3-/- mice when evaluated using SD-OCT, histological analyses, electron microscopy, scotopic electroretinogram, optokinetic response, and axonal anterograde transport. The lack of noticeable retinal abnormalities in F3-/- mice was confirmed in a human patient with biallelic loss-of-function mutations in F3. These data suggest that (i) F3 is important for maintaining the structural integrity of the cornea, (ii) absence of F3 does not affect the structure or function of any other ocular tissue in which it is expressed, and (iii) targeted silencing of F3 in the retina and/or RPE will likely be well-tolerated, serving as a safe therapeutic strategy for reducing sub-RPE deposit formation in disease. KEY MESSAGES: • Fibulins are expressed throughout the body at varying levels. • Fibulin-3 has a tissue-specific pattern of expression within the eye. • Lack of fibulin-3 leads to structural deformities in the cornea. • The retina and RPE remain structurally and functionally healthy in the absence of fibulin-3 in both mice and humans.
Keywords: Age-related macular degeneration (AMD); Cornea; EFEMP1; Fibulin-3; Malattia Leventinese (ML); Retina.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflicts of interest.
Figures

References
-
- Hulleman JD (2016) Malattia Leventinese/Doyne honeycomb retinal dystrophy: similarities to age-related macular degeneration and potential therapies. Adv Exp Med Biol 854:153–158 - PubMed
-
- McLaughlin PJ, Bakall B, Choi J, Liu Z, Sasaki T, Davis EC, Marmorstein AD, Marmorstein LY (2007) Lack of fibulin-3 causes early aging and herniation, but not macular degeneration in mice. Hum Mol Genet 16:3059–3070 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

