Administration of all-trans retinoic acid after experimental traumatic brain injury is brain protective
- PMID: 32964418
- PMCID: PMC7588818
- DOI: 10.1111/bph.15259
Administration of all-trans retinoic acid after experimental traumatic brain injury is brain protective
Abstract
Background and purpose: All-trans retinoic acid (ATRA) is a vitamin A metabolite, important in the developing and mature brain. Pre-injury ATRA administration ameliorates ischaemic brain insults in rodents. This study examined the effects of post-traumatic ATRA treatment in experimental traumatic brain injury (TBI).
Experimental approach: Male adult mice were subjected to the controlled cortical impact model of TBI or sham procedure and killed at 7 or 30 days post-injury (dpi). ATRA (10 mg kg-1, i.p.) was given immediately after the injury and 1, 2 and 3 dpi. Neurological function and sensorimotor coordination were evaluated. Brains were processed for (immuno-) histological, mRNA and protein analyses (qPCR and western blot).
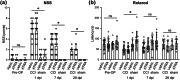
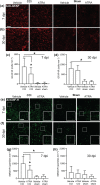
Key results: ATRA treatment reduced brain lesion size, reactive astrogliosis and axonal injury at 7 dpi, and hippocampal granule cell layer (GCL) integrity was protected at 7 and 30 dpi, independent of cell proliferation in neurogenic niches and blood-brain barrier damage. Neurological and motor deficits over time and the brain tissue loss at 30 dpi were not affected by ATRA treatment. ATRA decreased gene expression of markers for damage-associated molecular pattern (HMGB1), apoptosis (caspase-3 and Bax), activated microglia (TSPO), and reactive astrogliosis (GFAP, SerpinA3N) at 7 dpi and a subset of markers at 30 dpi (TSPO, GFAP).
Conclusion and implications: In experimental TBI, post-traumatic ATRA administration exerted brain protective effects, including long-term protection of GCL integrity, but did not affect neurological and motor deficits. Further investigations are required to optimize treatment regimens to enhance ATRA's brain protective effects and improve outcomes.
Keywords: all-trans retinoic acid; apoptosis; astrogliosis; axonal injury; hippocampus; neuroinflammation; traumatic brain injury.
© 2020. The British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Depletion of regulatory T cells increases T cell brain infiltration, reactive astrogliosis, and interferon-γ gene expression in acute experimental traumatic brain injury.J Neuroinflammation. 2019 Aug 5;16(1):163. doi: 10.1186/s12974-019-1550-0. J Neuroinflammation. 2019. PMID: 31383034 Free PMC article.
-
All-trans Retinoic Acid has Limited Therapeutic Effects on Cognition and Hippocampal Protein Expression After Controlled Cortical Impact.Neuroscience. 2022 Sep 1;499:130-141. doi: 10.1016/j.neuroscience.2022.07.021. Epub 2022 Jul 22. Neuroscience. 2022. PMID: 35878718
-
Single intracerebroventricular progranulin injection adversely affects the blood-brain barrier in experimental traumatic brain injury.J Neurochem. 2021 Jul;158(2):342-357. doi: 10.1111/jnc.15375. Epub 2021 May 12. J Neurochem. 2021. PMID: 33899947
-
Early posttraumatic CSF1R inhibition via PLX3397 leads to time- and sex-dependent effects on inflammation and neuronal maintenance after traumatic brain injury in mice.Brain Behav Immun. 2022 Nov;106:49-66. doi: 10.1016/j.bbi.2022.07.164. Epub 2022 Aug 3. Brain Behav Immun. 2022. PMID: 35933030
-
The Contribution of Hippocampal All-Trans Retinoic Acid (ATRA) Deficiency to Alzheimer's Disease: A Narrative Overview of ATRA-Dependent Gene Expression in Post-Mortem Hippocampal Tissue.Antioxidants (Basel). 2023 Oct 27;12(11):1921. doi: 10.3390/antiox12111921. Antioxidants (Basel). 2023. PMID: 38001775 Free PMC article. Review.
Cited by
-
Neuromelanin-induced cellular stress and neurotoxicity in the pathogenesis of Parkinson's disease.Apoptosis. 2025 Aug 7. doi: 10.1007/s10495-025-02156-3. Online ahead of print. Apoptosis. 2025. PMID: 40775594 Review.
-
Hippocampal Expression of Cytochrome P450 1B1 in Penetrating Traumatic Brain Injury.Int J Mol Sci. 2022 Jan 10;23(2):722. doi: 10.3390/ijms23020722. Int J Mol Sci. 2022. PMID: 35054909 Free PMC article.
-
Phosphatidylethanolamine Deficiency and Triglyceride Overload in Perilesional Cortex Contribute to Non-Goal-Directed Hyperactivity after Traumatic Brain Injury in Mice.Biomedicines. 2022 Apr 15;10(4):914. doi: 10.3390/biomedicines10040914. Biomedicines. 2022. PMID: 35453664 Free PMC article.
-
Microenvironmental Variations After Blood-Brain Barrier Breakdown in Traumatic Brain Injury.Front Mol Neurosci. 2021 Nov 26;14:750810. doi: 10.3389/fnmol.2021.750810. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34899180 Free PMC article. Review.
-
Valproic Acid Treatment after Traumatic Brain Injury in Mice Alleviates Neuronal Death and Inflammation in Association with Increased Plasma Lysophosphatidylcholines.Cells. 2024 Apr 23;13(9):734. doi: 10.3390/cells13090734. Cells. 2024. PMID: 38727269 Free PMC article.
References
-
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175(3), 407–411. 10.1111/bph.14112 - DOI - PMC - PubMed
-
- Beckers, L. , Ory, D. , Geric, I. , Declercq, L. , Koole, M. , Kassiou, M. , … Baes, M. (2018). Increased expression of translocator protein (TSPO) marks pro‐inflammatory microglia but does not predict neurodegeneration. Molecular Imaging and Biology: MIB: The Official Publication of the Academy of Molecular Imaging, 20, 94–102. 10.1007/s11307-017-1099-1 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

