Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB pathways
- PMID: 32964428
- PMCID: PMC7589024
- DOI: 10.1111/bph.15261
Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB pathways
Abstract
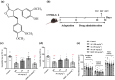
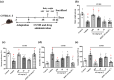
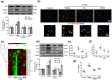
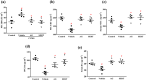
Background and purpose: Arctigenin, a major bioactive component of Fructus arctii, has been reported to have antidepressant-like effects. However, the mechanisms underlying these effects are still unclear. Neuroinflammation can be caused by excessive production of proinflammatory cytokines in microglia via high-mobility group box 1 (HMGB1)/TLR4/NF-κB and TNF-α/TNFR1/NF-κB signalling pathways, leading to depression. In this study, we have investigated the antidepressant mechanism of arctigenin by conducting in vitro and in vivo studies.
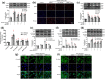
Experimental approach: The effects of chronic unpredictable mild stress (CUMS) on wild-type (WT) and TLR4-/- mice were examined. Antidepressant-like effects of arctigenin were tested using the CUMS-induced model of depression in WT mice. The effects of arctigenin were assessed on the HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB signalling pathways in the prefrontal cortex (PFC) of mouse brain and HMGB1- or TNF-α-stimulated primary cultured microglia. The interaction between HMGB1 and TLR4 or TNF-α and TNFR1 with or without arctigenin was examined by localized surface plasmon resonance (LSPR) and co-immunoprecipitation assays.
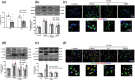
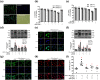
Key results: The immobility times in the tail suspension test (TST) and forced swimming test (FST) were reduced in TLR4-/- mice, compared with WT mice. Arctigenin exhibited antidepressant-like effects. Arctigenin also inhibited microglia activation and inflammatory responses in the PFC of mouse brain. Arctigenin inhibited HMGB1 and TLR4 or TNF-α and TNFR1 interactions, and suppressed both HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB signalling pathways.
Conclusions and implications: Arctigenin has antidepressant-like effects by attenuating excessive microglial activation and neuroinflammation through the HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB signalling pathways. This suggests that arctigenin has potential as a new drug candidate suitable for clinical trials to treat depression.
Keywords: HMGB1; TNF-α; antidepressant; arctigenin; microglia; neuroinflammation.
© 2020. The British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , … CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: Introduction and other protein targets. British Journal of Pharmacology, 176(Suppl 1), S1–S20. 10.1111/bph.14747 - DOI - PMC - PubMed
-
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology . British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

