Activation of neuronal Ras-related C3 botulinum toxin substrate 1 (Rac1) improves post-stroke recovery and axonal plasticity in mice
- PMID: 32964455
- PMCID: PMC7982352
- DOI: 10.1111/jnc.15195
Activation of neuronal Ras-related C3 botulinum toxin substrate 1 (Rac1) improves post-stroke recovery and axonal plasticity in mice
Abstract
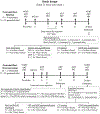
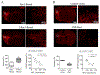
Long-term disability after stroke is common but the mechanisms of post-stroke recovery remain unclear. Cerebral Ras-related C3 botulinum toxin substrate (Rac) 1 contributes to functional recovery after ischemic stroke in mice. As Rac1 plays divergent roles in individual cell types after central neural system injury, we herein examined the specific role of neuronal Rac1 in post-stroke recovery and axonal regeneration. Young male mice were subjected to 60-min of middle cerebral artery occlusion (MCAO). Inducible deletion of neuronal Rac1 by daily intraperitoneal injection of tamoxifen (2 mg/40 g) into Thy1-creER/Rac1-floxed mice day 7-11 after MCAO worsened cognitive (assayed by novel object recognition test) and sensorimotor (assayed by adhesive removal and pellet reaching tests) recovery day 14-28 accompanied with the reduction of neurofilament-L (NFL) and myelin basic protein (MBP) and the elevation of glial fibrillary acidic protein (GFAP) in the peri-infarct zone assessed by immunostaining. Whereas the brain tissue loss was not altered assayed by cresyl violet staining. In another approach, delayed overexpression of neuronal Rac1 by injection of lentivirus encoding Rac1 with neuronal promotor into both the cortex and striatum (total 4 μl at 1 × 109 transducing units/mL) of stroke side in C57BL/6J mice day 7 promoted stroke outcome, NFL and MBP regrowth and alleviated GFAP invasion. Furthermore, neuronal Rac1 over-expression led to the activation of p21 activating kinases (PAK) 1, mitogen-activated protein kinase kinase (MEK) 1/2 and extracellular signal-regulated kinase (ERK) 1/2, and the elevation of brain-derived neurotrophic factor (BDNF) day 14 after stroke. Finally, we observed higher counts of neuronal Rac1 in the peri-infarct zone of subacute/old ischemic stroke subjects. This work identified a neuronal Rac1 signaling in improving functional recovery and axonal regeneration after stroke, suggesting a potential therapeutic target in the recovery stage of stroke.
Keywords: Rac1; functional recovery; glia scar formation; ischemic stroke; neural plasticity.
© 2020 International Society for Neurochemistry.
Conflict of interest statement
Disclosures
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures






Similar articles
-
Activation of endothelial ras-related C3 botulinum toxin substrate 1 (Rac1) improves post-stroke recovery and angiogenesis via activating Pak1 in mice.Exp Neurol. 2019 Dec;322:113059. doi: 10.1016/j.expneurol.2019.113059. Epub 2019 Sep 6. Exp Neurol. 2019. PMID: 31499064 Free PMC article. Review.
-
Activation of cerebral Ras-related C3 botulinum toxin substrate (Rac) 1 promotes post-ischemic stroke functional recovery in aged mice.Neural Regen Res. 2024 Apr;19(4):881-886. doi: 10.4103/1673-5374.382256. Neural Regen Res. 2024. PMID: 37843224 Free PMC article.
-
Ras-Related C3 Botulinum Toxin Substrate 1 Promotes Axonal Regeneration after Stroke in Mice.Transl Stroke Res. 2018 Oct;9(5):506-514. doi: 10.1007/s12975-018-0611-5. Epub 2018 Feb 24. Transl Stroke Res. 2018. PMID: 29476448 Free PMC article.
-
Neuronal Rho GTPase Rac1 elimination confers neuroprotection in a mouse model of permanent ischemic stroke.Brain Pathol. 2018 Jul;28(4):569-580. doi: 10.1111/bpa.12562. Epub 2017 Oct 24. Brain Pathol. 2018. PMID: 28960571 Free PMC article.
-
Role of Rac1-mineralocorticoid-receptor signalling in renal and cardiac disease.Nat Rev Nephrol. 2013 Feb;9(2):86-98. doi: 10.1038/nrneph.2012.282. Epub 2013 Jan 8. Nat Rev Nephrol. 2013. PMID: 23296296 Review.
Cited by
-
Non-invasive Vagus Nerve Stimulation in Cerebral Stroke: Current Status and Future Perspectives.Front Neurosci. 2022 Feb 16;16:820665. doi: 10.3389/fnins.2022.820665. eCollection 2022. Front Neurosci. 2022. PMID: 35250458 Free PMC article. Review.
-
PAK1 contributes to cerebral ischemia/reperfusion injury by regulating the blood-brain barrier integrity.iScience. 2023 Jul 11;26(8):107333. doi: 10.1016/j.isci.2023.107333. eCollection 2023 Aug 18. iScience. 2023. PMID: 37529106 Free PMC article.
-
Ginsenoside Rg1 promotes neurite growth of retinal ganglion cells through cAMP/PKA/CREB pathways.J Ginseng Res. 2024 Mar;48(2):163-170. doi: 10.1016/j.jgr.2022.05.002. Epub 2022 May 24. J Ginseng Res. 2024. PMID: 38465221 Free PMC article.
-
Vagus nerve stimulation in cerebral stroke: biological mechanisms, therapeutic modalities, clinical applications, and future directions.Neural Regen Res. 2024 Aug 1;19(8):1707-1717. doi: 10.4103/1673-5374.389365. Epub 2023 Nov 8. Neural Regen Res. 2024. PMID: 38103236 Free PMC article.
-
Integrated bioinformatics analysis and biological experiments to identify key immune genes in vascular dementia.Front Immunol. 2025 Mar 24;16:1560438. doi: 10.3389/fimmu.2025.1560438. eCollection 2025. Front Immunol. 2025. PMID: 40196107 Free PMC article.
References
-
- Bevins RA and Besheer J (2006) Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc 1, 1306–1311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

