Automatic contouring system for cervical cancer using convolutional neural networks
- PMID: 32964477
- PMCID: PMC7756586
- DOI: 10.1002/mp.14467
Automatic contouring system for cervical cancer using convolutional neural networks
Abstract
Purpose: To develop a tool for the automatic contouring of clinical treatment volumes (CTVs) and normal tissues for radiotherapy treatment planning in cervical cancer patients.
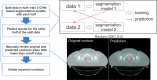
Methods: An auto-contouring tool based on convolutional neural networks (CNN) was developed to delineate three cervical CTVs and 11 normal structures (seven OARs, four bony structures) in cervical cancer treatment for use with the Radiation Planning Assistant, a web-based automatic plan generation system. A total of 2254 retrospective clinical computed tomography (CT) scans from a single cancer center and 210 CT scans from a segmentation challenge were used to train and validate the CNN-based auto-contouring tool. The accuracy of the tool was evaluated by calculating the Sørensen-dice similarity coefficient (DSC) and mean surface and Hausdorff distances between the automatically generated contours and physician-drawn contours on 140 internal CT scans. A radiation oncologist scored the automatically generated contours on 30 external CT scans from three South African hospitals.
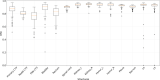
Results: The average DSC, mean surface distance, and Hausdorff distance of our CNN-based tool were 0.86/0.19 cm/2.02 cm for the primary CTV, 0.81/0.21 cm/2.09 cm for the nodal CTV, 0.76/0.27 cm/2.00 cm for the PAN CTV, 0.89/0.11 cm/1.07 cm for the bladder, 0.81/0.18 cm/1.66 cm for the rectum, 0.90/0.06 cm/0.65 cm for the spinal cord, 0.94/0.06 cm/0.60 cm for the left femur, 0.93/0.07 cm/0.66 cm for the right femur, 0.94/0.08 cm/0.76 cm for the left kidney, 0.95/0.07 cm/0.84 cm for the right kidney, 0.93/0.05 cm/1.06 cm for the pelvic bone, 0.91/0.07 cm/1.25 cm for the sacrum, 0.91/0.07 cm/0.53 cm for the L4 vertebral body, and 0.90/0.08 cm/0.68 cm for the L5 vertebral bodies. On average, 80% of the CTVs, 97% of the organ at risk, and 98% of the bony structure contours in the external test dataset were clinically acceptable based on physician review.
Conclusions: Our CNN-based auto-contouring tool performed well on both internal and external datasets and had a high rate of clinical acceptability.
Keywords: auto-contouring; cervical cancer; convolutional neural network; deep learning.
© 2020 The Authors. Medical Physics published by Wiley Periodicals LLC on behalf of American Association of Physicists in Medicine.
Conflict of interest statement
This work was partially funded by the National Cancer Institute and Varian Medical Systems.
Figures






Similar articles
-
Evaluation of auto-segmentation for EBRT planning structures using deep learning-based workflow on cervical cancer.Sci Rep. 2022 Aug 11;12(1):13650. doi: 10.1038/s41598-022-18084-0. Sci Rep. 2022. PMID: 35953516 Free PMC article.
-
Automatic detection of contouring errors using convolutional neural networks.Med Phys. 2019 Nov;46(11):5086-5097. doi: 10.1002/mp.13814. Epub 2019 Sep 26. Med Phys. 2019. PMID: 31505046 Free PMC article.
-
Self-configuring nnU-Net for automatic delineation of the organs at risk and target in high-dose rate cervical brachytherapy, a low/middle-income country's experience.J Appl Clin Med Phys. 2023 Aug;24(8):e13988. doi: 10.1002/acm2.13988. Epub 2023 Apr 12. J Appl Clin Med Phys. 2023. PMID: 37042449 Free PMC article.
-
Deep learning-based auto-segmentation of clinical target volumes for radiotherapy treatment of cervical cancer.J Appl Clin Med Phys. 2022 Feb;23(2):e13470. doi: 10.1002/acm2.13470. Epub 2021 Nov 22. J Appl Clin Med Phys. 2022. PMID: 34807501 Free PMC article.
-
Automated Contouring and Planning in Radiation Therapy: What Is 'Clinically Acceptable'?Diagnostics (Basel). 2023 Feb 10;13(4):667. doi: 10.3390/diagnostics13040667. Diagnostics (Basel). 2023. PMID: 36832155 Free PMC article. Review.
Cited by
-
Multi-organ segmentation of abdominal structures from non-contrast and contrast enhanced CT images.Sci Rep. 2022 Nov 9;12(1):19093. doi: 10.1038/s41598-022-21206-3. Sci Rep. 2022. PMID: 36351987 Free PMC article.
-
Cervical cancer segmentation based on medical images: a literature review.Quant Imaging Med Surg. 2024 Jul 1;14(7):5176-5204. doi: 10.21037/qims-24-369. Epub 2024 Jun 11. Quant Imaging Med Surg. 2024. PMID: 39022282 Free PMC article. Review.
-
A Feasibility Study of Deep Learning-Based Auto-Segmentation Directly Used in VMAT Planning Design and Optimization for Cervical Cancer.Front Oncol. 2022 Jun 1;12:908903. doi: 10.3389/fonc.2022.908903. eCollection 2022. Front Oncol. 2022. PMID: 35719942 Free PMC article.
-
Evaluation of auto-segmentation for EBRT planning structures using deep learning-based workflow on cervical cancer.Sci Rep. 2022 Aug 11;12(1):13650. doi: 10.1038/s41598-022-18084-0. Sci Rep. 2022. PMID: 35953516 Free PMC article.
-
Impact of annotation imperfections and auto-curation for deep learning-based organ-at-risk segmentation.Phys Imaging Radiat Oncol. 2024 Dec 4;32:100684. doi: 10.1016/j.phro.2024.100684. eCollection 2024 Oct. Phys Imaging Radiat Oncol. 2024. PMID: 39720784 Free PMC article.
References
-
- Vorwerk H, Zink K, Schiller R, et al. Protection of quality and innovation in radiation oncology: the prospective multicenter trial the German Society of Radiation Oncology (DEGRO‐QUIRO study). Strahlentherapie und Onkol. 2014;190:433–443. - PubMed
-
- Andrianarison VA, Laouiti M, Fargier‐Bochaton O, et al. Contouring workload in adjuvant breast cancer radiotherapy. Cancer Radiother. 2018;22:747–753. - PubMed
-
- Yang J, Zhang Y, Zhang L, Dong L. Automatic segmentation of parotids from CT scans using multiple atlases Medical Image Analysis for the Clinic: A Grand Challenge. 2010:223–230.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

