Nutrient metabolism and cancer in the in vivo context: a metabolic game of give and take
- PMID: 32964587
- PMCID: PMC7534637
- DOI: 10.15252/embr.202050635
Nutrient metabolism and cancer in the in vivo context: a metabolic game of give and take
Abstract
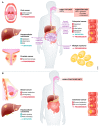
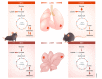
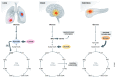
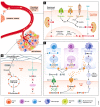
Nutrients are indispensable resources that provide the macromolecular building blocks and energy requirements for sustaining cell growth and survival. Cancer cells require several key nutrients to fulfill their changing metabolic needs as they progress through stages of development. Moreover, both cell-intrinsic and microenvironment-influenced factors determine nutrient dependencies throughout cancer progression-for which a comprehensive characterization remains incomplete. In addition to the widely studied role of genetic alterations driving cancer metabolism, nutrient use in cancer tissue may be affected by several factors including the following: (i) diet, the primary source of bodily nutrients which influences circulating metabolite levels; (ii) tissue of origin, which can influence the tumor's reliance on specific nutrients to support cell metabolism and growth; (iii) local microenvironment, which dictates the accessibility of nutrients to tumor cells; (iv) tumor heterogeneity, which promotes metabolic plasticity and adaptation to nutrient demands; and (v) functional demand, which intensifies metabolic reprogramming to fuel the phenotypic changes required for invasion, growth, or survival. Here, we discuss the influence of these factors on nutrient metabolism and dependence during various steps of tumor development and progression.
Keywords: cancer metabolism; diet; microenvironment; nutrients; tumor heterogeneity.
© 2020 The Authors.
Conflict of interest statement
S.‐M.F. has received funding from Bayer, Merck, and Black Belt Therapeutics and has consulted for Fund+. The other authors have no conflict of interest to declare.
Figures

References
-
- Aguirre‐Ghiso JA, Sosa MS (2018) Emerging topics on disseminated cancer cell dormancy and the paradigm of metastasis. Annu Rev Cancer Biol 2: 377–393
-
- Ahmed N, Escalona R, Leung D, Chan E, Kannourakis G (2018) Tumour microenvironment and metabolic plasticity in cancer and cancer stem cells: perspectives on metabolic and immune regulatory signatures in chemoresistant ovarian cancer stem cells. Semin Cancer Biol 53: 265–281 - PubMed
-
- Alix‐Panabières C, Cayrefourcq L, Mazard T, Maudelonde T, Assenat E, Assou S (2020) Molecular Portrait of Metastasis‐Competent Circulating Tumor Cells in Colon Cancer Reveals the Crucial Role of Genes Regulating Energy Metabolism and DNA Repair. Clin Chem 63: 700–713 - PubMed
-
- Andrzejewski S, Klimcakova E, Johnson RM, Tabariès S, Annis MG, McGuirk S, Northey JJ, Chénard V, Sriram U, Papadopoli DJ et al (2017) PGC‐1α Promotes Breast Cancer Metastasis and Confers Bioenergetic Flexibility against Metabolic Drugs. Cell Metab 26: 778–787.e5 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 839896/EC|H2020|H2020 Priority Excellent Science|H2020 Marie Skłodowska-Curie Actions (MSCA)
- 771486/EC|H2020|H2020 Priority Excellent Science|H2020 European Research Council (ERC)
- Boehringer Ingelheim Fonds (BIF)
- G098120N/Fonds Wetenschappelijk Onderzoek (FWO)
- G088318N/Fonds Wetenschappelijk Onderzoek (FWO)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

