Immune response following infection with SARS-CoV-2 and other coronaviruses: A rapid review
- PMID: 32964627
- PMCID: PMC7536965
- DOI: 10.1002/rmv.2162
Immune response following infection with SARS-CoV-2 and other coronaviruses: A rapid review
Abstract
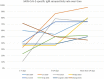
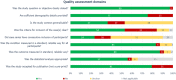
In this review, we systematically searched and summarized the evidence on the immune response and reinfection rate following SARS-CoV-2 infection. We also retrieved studies on SARS-CoV and MERS-CoV to assess the long-term duration of antibody responses. A protocol based on Cochrane rapid review methodology was adhered to and databases were searched from 1/1/2000 until 26/5/2020. Of 4744 citations retrieved, 102 studies met our inclusion criteria. Seventy-four studies were retrieved on SARS-CoV-2. While the rate and timing of IgM and IgG seroconversion were inconsistent across studies, most seroconverted for IgG within 2 weeks and 100% (N = 62) within 4 weeks. IgG was still detected at the end of follow-up (49-65 days) in all patients (N = 24). Neutralizing antibodies were detected in 92%-100% of patients (up to 53 days). It is not clear if reinfection with SARS-CoV-2 is possible, with studies more suggestive of intermittent detection of residual RNA. Twenty-five studies were retrieved on SARS-CoV. In general, SARS-CoV-specific IgG was maintained for 1-2 years post-infection and declined thereafter, although one study detected IgG up to 12 years post-infection. Neutralizing antibodies were detected up to 17 years in another study. Three studies on MERS-CoV reported that IgG may be detected up to 2 years. In conclusion, limited early data suggest that most patients seroconvert for SARS-CoV-2-specific IgG within 2 weeks. While the long-term duration of antibody responses is unknown, evidence from SARS-CoV studies suggest SARS-CoV-specific IgG is sustained for 1-2 years and declines thereafter.
Keywords: COVID-19; MERS-CoV; SARS-CoV; SARS-CoV-2; seasonal coronaviruses.
© 2020 John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no competing interest.
Figures


References
-
- HIQA. Health Information and Quality Authority . Protocol for evidence synthesis support ‐ COVID‐19. https://www.hiqa.ie/sites/default/files/2020‐05/Protocol‐for‐HIQA‐COVID‐.... 2020. Accessed June 1, 2020.
-
- Garritty C, Gartlehner G, Kamel C et al. Cochrane Rapid Reviews. Interim Guidance from the Cochrane Rapid Reviews Methods Group; March 2020.
-
- Wells GA, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed August 14, 2020.
-
- Adams ER, Anand R, Andersson MI, et al. Evaluation of antibody testing for SARS‐Cov‐2 using ELISA and lateral flow immunoassays. medRxiv. 2020. doi:10.1101/2020.04.15.20066407. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

