Tumor heterogeneity estimation for radiomics in cancer
- PMID: 32964647
- PMCID: PMC8244619
- DOI: 10.1002/sim.8749
Tumor heterogeneity estimation for radiomics in cancer
Abstract
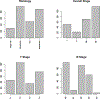
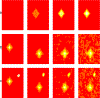
Radiomics is an emerging field of medical image analysis research where quantitative measurements are obtained from radiological images that can be utilized to predict patient outcomes and inform treatment decisions. Cancer patients routinely undergo radiological evaluations when images of various modalities including computed tomography, positron emission tomography, and magnetic resonance images are collected for diagnosis and for evaluation of disease progression. Tumor characteristics, often referred to as measures of tumor heterogeneity, can be computed using these clinical images and used as predictors of disease progression and patient survival. Several approaches for quantifying tumor heterogeneity have been proposed, including intensity histogram-based measures, shape and volume-based features, and texture analysis. Taking into account the topology of the tumors we propose a statistical framework for estimating tumor heterogeneity using clustering based on Markov random field theory. We model the voxel intensities using a Gaussian mixture model using a Gibbs prior to incorporate voxel neighborhood information. We propose a novel approach to choosing the number of mixture components. Subsequently, we show that the proposed procedure outperforms the existing approaches when predicting lung cancer survival.
Keywords: Markov random fields; cancer imaging; computed tomography; image segmentation; machine learning.
© 2020 John Wiley & Sons Ltd.
Figures





References
-
- Aerts H, Rios Velazquez E, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, and Lambin P. Data from nsclc-radiomics. Cancer Imaging Archive, 2015.
-
- Andersen PK and Gill RD. Cox’s regression model for counting processes: a large sample study. The annals of statistics, pages 1100–1120, 1982.
-
- Binder H, Allignol A, Schumacher M, and Beyersmann J. Boosting for high-dimensional time-to-event data with competing risks. Bioinformatics, 25(7):890–896, 2009. - PubMed
-
- Binder H, Benner A, Bullinger L, and Schumacher M. Tailoring sparse multivariable regression techniques for prognostic single-nucleotide polymorphism signatures. Statistics in medicine, 32(10):1778–1791, 2013. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
