Synthetic Antibody Mimics Based on Cancer-Targeting Immunostimulatory Peptides
- PMID: 32964656
- PMCID: PMC8191480
- DOI: 10.1002/cbic.202000407
Synthetic Antibody Mimics Based on Cancer-Targeting Immunostimulatory Peptides
Abstract
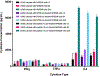
De novo cancer-targeting immunostimulatory peptides have been designed and developed as synthetic antibody mimics. A series of bifunctional peptides incorporating NKp30-binding and NK-cell-activating domains were synthesized as linear dimers and then extended into branching trimeric peptides by the incorporation of GRP78-targeting and tumor-cell-binding sequences. A selected trimeric peptide from this small set of peptides displayed binding capabilities on GRP78+ HepG2 and A549 target cells. Cell binding diminished in the presence of an anti-GRP78 peptide blocker, thus suggesting GRP78-binding dependence. Similarly, the selected trimeric peptide was also found to exhibit NK cell binding in an NKp30-dependent manner, which translated into NK cell activation as indicated by cytokine secretion. In co-culture, fluorescence microscopy revealed that the target GFP-expressing A549 cells were visibly associated with the effector NK cells when pre-activated with lead trimeric peptide. Accordingly, A549 cells were found to be compromised, as evidenced by the loss of GFP signal and notable detection of early-/late-stage apoptosis. Investigation of the immunological markers related to toxicity revealed detectable secretion of pro-inflammatory cytokines and chemokines, including IFN-γ, TNF-α, and IL-8. Furthermore, administration of peptide-activated NK cells into A549-tumor-bearing mice resulted in a consistent decrease in tumor growth when compared to the untreated control group. Taken together, the identification of a lead trimeric peptide capable of targeting and activating NK cells' immunotoxicity directly towards GRP78+ /B7H6- tumors provides a novel proof-of-concept for the development of cancer-targeting immunostimulatory peptide ligands that mimic antibody-targeting and -activating functions related to cancer immunotherapy applications.
Keywords: cancer immunotherapy; cancer-targeting peptides; immunostimulatory peptides; peptide vaccines; synthetic antibody mimics.
© 2020 Wiley-VCH GmbH.
Conflict of interest statement
Conflicts of Interest
The authors declare no competing financial interest.
Figures

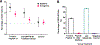



References
-
- Lesterhuis WJ, Haanen JBAG, Punt CJA, Nature Rev. Drug Discov. 2011, 10, 591–600. - PubMed
-
- Kimiz-Gebologlu I, Gulce-Iz S, Biray-Avci C, Mol. Biol. Rep 2018, 45, 2935–2940. - PubMed
-
- Guillerey C, Huntington ND, Smyth MJ, Nat MJ. Immunol. 2016, 17, 1025–1036. - PubMed
-
- Ljunggren HG, Malmberg KJ, Nat KJ. Rev. Immunol 2007, 7, 329–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

