Klotho overexpression improves amyloid-β clearance and cognition in the APP/PS1 mouse model of Alzheimer's disease
- PMID: 32964663
- PMCID: PMC7576297
- DOI: 10.1111/acel.13239
Klotho overexpression improves amyloid-β clearance and cognition in the APP/PS1 mouse model of Alzheimer's disease
Abstract
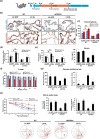
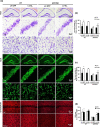
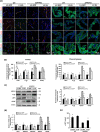
Alzheimer's disease (AD) is the most prevalent type of dementia, characterized by the presence of amyloid-β (Aβ) plaques. We previously reported that Klotho lowered Aβ levels in the brain and protected against cognitive deficits in amyloid precursor protein/presenilin 1(APP/PS1) mice. However, the underlying mechanism remains unclear. In this study, we induced intracerebral Klotho overexpression in 13-month-old APP/PS1 mice by injecting lentivirus that carried full-length mouse Klotho cDNA in the lateral ventricle of the brain. We examined the effects of Klotho overexpression on cognition, Aβ burden, Aβ-related neuropathology, microglia transformation, and Aβ transport systems in vivo. Additionally, we investigated the effects of Klotho on Aβ transport at the blood-cerebrospinal fluid barrier by knocking down Klotho in primary human choroid plexus epithelial cells (HCPEpiCs). The upregulation of Klotho levels in the brain and serum significantly ameliorated Aβ burden, neuronal and synaptic loss and cognitive deficits in aged APP/PS1 mice. Klotho treatment significantly inhibited NACHT, LRR, and PYD domain-containing protein 3 (NLRP3) and the subsequent transformation of microglia to the M2 type that may enhance microglia-mediated Aβ clearance. Meanwhile, Klotho overexpression also regulated Aβ transporter expression, which may promote Aβ transporter-mediated Aβ clearance. Moreover, the ability of HCPEpiCs to transport Aβ in vitro was also significantly impaired by Klotho knockdown. Given the neuroprotective effect of Klotho overexpression, the present findings suggest that Klotho should be further investigated as a potential therapeutic target for AD.
Keywords: Alzheimer's disease; Aβ clearance; Klotho.
© 2020 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interests.
Figures



References
-
- Baluchnejadmojarad, T. , Eftekhari, S.‐M. , Jamali‐Raeufy, N. , Haghani, S. , Zeinali, H. , & Roghani, M. (2017). The anti‐aging protein klotho alleviates injury of nigrostriatal dopaminergic pathway in 6‐hydroxydopamine rat model of Parkinson's disease: Involvement of PKA/CaMKII/CREB signaling. Experimental Gerontology, 100, 70–76. 10.1016/j.exger.2017.10.023 - DOI - PubMed
-
- Chiu, C. , Miller, M. C. , Caralopoulos, I. N. , Worden, M. S. , Brinker, T. , Gordon, Z. N. , … Silverberg, G. D. (2012). Temporal course of cerebrospinal fluid dynamics and amyloid accumulation in the aging rat brain from three to thirty months. Fluids and Barriers of the CNS, 9(1), 3 10.1186/2045-8118-9-3 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

