Tissue dynamics spectroscopic imaging: functional imaging of heterogeneous cancer tissue
- PMID: 32964703
- PMCID: PMC7506185
- DOI: 10.1117/1.JBO.25.9.096006
Tissue dynamics spectroscopic imaging: functional imaging of heterogeneous cancer tissue
Abstract
Significance: Tumor heterogeneity poses a challenge for the chemotherapeutic treatment of cancer. Tissue dynamics spectroscopy captures dynamic contrast and can capture the response of living tissue to applied therapeutics, but the current analysis averages over the complicated spatial response of living biopsy samples.
Aim: To develop tissue dynamics spectroscopic imaging (TDSI) to map the heterogeneous spatial response of tumor tissue to anticancer drugs.
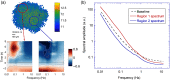
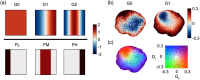
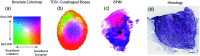
Approach: TDSI is applied to tumor spheroids grown from cell lines and to ex vivo living esophageal biopsy samples. Doppler fluctuation spectroscopy is performed on a voxel basis to extract spatial maps of biodynamic biomarkers. Functional images and bivariate spatial maps are produced using a bivariate color merge to represent the spatial distribution of pairs of signed drug-response biodynamic biomarkers.
Results: We have mapped the spatial variability of drug responses within biopsies and have tracked sample-to-sample variability. Sample heterogeneity observed in the biodynamic maps is associated with histological heterogeneity observed using inverted selective-plane illumination microscopy.
Conclusion: We have demonstrated the utility of TDSI as a functional imaging method to measure tumor heterogeneity and its potential for use in drug-response profiling.
Keywords: deep optical imaging; functional imaging; tissue-dynamics imaging; tumor spatial heterogeneity.
Figures





Similar articles
-
Common-path interferometer for digital holographic Doppler spectroscopy of living biological tissues.J Biomed Opt. 2021 Mar;26(3):030501. doi: 10.1117/1.JBO.26.3.030501. J Biomed Opt. 2021. PMID: 33783149 Free PMC article.
-
Biodynamic imaging for phenotypic profiling of three-dimensional tissue culture.J Biomed Opt. 2017 Jan 1;22(1):16007. doi: 10.1117/1.JBO.22.1.016007. J Biomed Opt. 2017. PMID: 28301634 Free PMC article.
-
Intracellular Doppler Signatures of Platinum Sensitivity Captured by Biodynamic Profiling in Ovarian Xenografts.Sci Rep. 2016 Jan 6;6:18821. doi: 10.1038/srep18821. Sci Rep. 2016. PMID: 26732545 Free PMC article.
-
Quantitative Clinical Imaging Methods for Monitoring Intratumoral Evolution.Methods Mol Biol. 2017;1513:61-81. doi: 10.1007/978-1-4939-6539-7_6. Methods Mol Biol. 2017. PMID: 27807831 Review.
-
[Mass spectrometry imaging technology and its application in breast cancer research].Se Pu. 2021 Jun;39(6):578-587. doi: 10.3724/SP.J.1123.2020.10005. Se Pu. 2021. PMID: 34227318 Free PMC article. Review. Chinese.
Cited by
-
Intracellular doppler spectroscopy of live tissue sentinels for a fast in-vitro bacterial infection assay.Sci Rep. 2025 Jul 5;15(1):24020. doi: 10.1038/s41598-025-08523-z. Sci Rep. 2025. PMID: 40617925 Free PMC article.
-
Doppler imaging detects bacterial infection of living tissue.Commun Biol. 2021 Feb 10;4(1):178. doi: 10.1038/s42003-020-01550-8. Commun Biol. 2021. PMID: 33568744 Free PMC article.
-
Comparative oncology chemosensitivity assay for personalized medicine using low-coherence digital holography of dynamic light scattering from cancer biopsies.Sci Rep. 2024 Feb 8;14(1):2760. doi: 10.1038/s41598-024-52404-w. Sci Rep. 2024. PMID: 38332203 Free PMC article.
-
Imaging and characterization of transitions in biofilm morphology via anomalous diffusion following environmental perturbation.Biomed Opt Express. 2022 Feb 23;13(3):1654-1670. doi: 10.1364/BOE.449131. eCollection 2022 Mar 1. Biomed Opt Express. 2022. PMID: 35414993 Free PMC article.
-
Common-path interferometer for digital holographic Doppler spectroscopy of living biological tissues.J Biomed Opt. 2021 Mar;26(3):030501. doi: 10.1117/1.JBO.26.3.030501. J Biomed Opt. 2021. PMID: 33783149 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

