Coronavirus interactions with the cellular autophagy machinery
- PMID: 32964796
- PMCID: PMC7755319
- DOI: 10.1080/15548627.2020.1817280
Coronavirus interactions with the cellular autophagy machinery
Abstract
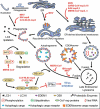
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, is the most recent example of an emergent coronavirus that poses a significant threat to human health. Virus-host interactions play a major role in the viral life cycle and disease pathogenesis, and cellular pathways such as macroautophagy/autophagy prove to be either detrimental or beneficial to viral replication and maturation. Here, we describe the literature over the past twenty years describing autophagy-coronavirus interactions. There is evidence that many coronaviruses induce autophagy, although some of these viruses halt the progression of the pathway prior to autophagic degradation. In contrast, other coronaviruses usurp components of the autophagy pathway in a non-canonical fashion. Cataloging these virus-host interactions is crucial for understanding disease pathogenesis, especially with the global challenge of SARS-CoV-2 and COVID-19. With the recognition of autophagy inhibitors, including the controversial drug chloroquine, as possible treatments for COVID-19, understanding how autophagy affects the virus will be critical going forward. Abbreviations: 3-MA: 3-methyladenine (autophagy inhibitor); AKT/protein kinase B: AKT serine/threonine kinase; ATG: autophagy related; ATPase: adenosine triphosphatase; BMM: bone marrow macrophage; CGAS: cyclic GMP-AMP synthase; CHO: Chinese hamster ovary/cell line; CoV: coronaviruses; COVID-19: Coronavirus disease 2019; DMV: double-membrane vesicle; EAV: equine arteritis virus; EDEM1: ER degradation enhancing alpha-mannosidase like protein 1; ER: endoplasmic reticulum; ERAD: ER-associated degradation; GFP: green fluorescent protein; HCoV: human coronavirus; HIV: human immunodeficiency virus; HSV: herpes simplex virus; IBV: infectious bronchitis virus; IFN: interferon; LAMP1: lysosomal associated membrane protein 1; MAP1LC3/LC3: microtubule associated protein 1 light chain 3; MCoV: mouse coronavirus; MERS-CoV: Middle East respiratory syndrome coronavirus; MHV: mouse hepatitis virus; NBR1: NBR1 autophagy cargo receptor; CALCOCO2/NDP52: calcium binding and coiled-coil domain 2 (autophagy receptor that directs cargo to phagophores); nsp: non-structural protein; OS9: OS9 endoplasmic reticulum lectin; PEDV: porcine epidemic diarrhea virus; PtdIns3K: class III phosphatidylinositol 3-kinase; PLP: papain-like protease; pMEF: primary mouse embryonic fibroblasts; SARS-CoV: severe acute respiratory syndrome coronavirus; SKP2: S-phase kinase associated protein 2; SQSTM1: sequestosome 1; STING1: stimulator of interferon response cGAMP interactor 1; ULK1: unc-51 like autophagy activating kinase 1; Vps: vacuolar protein sorting.
Keywords: Autophagy; COVID-19; ERAD; MERS; SARS-CoV-2; coronavirus.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures
References
-
- Fung TS, Liu DX. Human coronavirus: host-pathogen interaction. Annu Rev Microbiol. 2019;73:529–557. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
