Situating the left-lateralized language network in the broader organization of multiple specialized large-scale distributed networks
- PMID: 32965153
- PMCID: PMC8356783
- DOI: 10.1152/jn.00753.2019
Situating the left-lateralized language network in the broader organization of multiple specialized large-scale distributed networks
Abstract
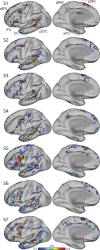
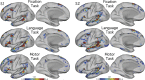
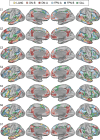
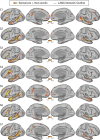
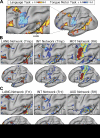
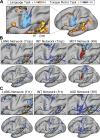
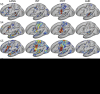
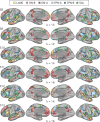
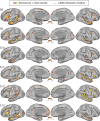
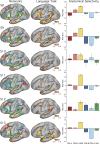
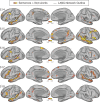
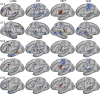
Using procedures optimized to explore network organization within the individual, the topography of a candidate language network was characterized and situated within the broader context of adjacent networks. The candidate network was first identified using functional connectivity and replicated across individuals, acquisition tasks, and analytical methods. In addition to classical language regions near the perisylvian cortex and temporal pole, regions were also observed in dorsal posterior cingulate, midcingulate, and anterior superior frontal and inferior temporal cortex. The candidate network was selectively activated when processing meaningful (as contrasted with nonword) sentences, whereas spatially adjacent networks showed minimal or even decreased activity. Results were replicated and triplicated across two prospectively acquired cohorts. Examined in relation to adjacent networks, the topography of the language network was found to parallel the motif of other association networks, including the transmodal association networks linked to theory of mind and episodic remembering (often collectively called the default network). The several networks contained juxtaposed regions in multiple association zones. Outside of these juxtaposed higher-order networks, we further noted a distinct frontotemporal network situated between language regions and a frontal orofacial motor region and a temporal auditory region. A possibility is that these functionally related sensorimotor regions might anchor specialization of neighboring association regions that develop into a language network. What is most striking is that the canonical language network appears to be just one of multiple similarly organized, differentially specialized distributed networks that populate the evolutionarily expanded zones of human association cortex.NEW & NOTEWORTHY This research shows that a language network can be identified within individuals using functional connectivity. Organizational details reveal that the language network shares a common spatial motif with other association networks, including default and frontoparietal control networks. The language network is activated by language task demands, whereas closely juxtaposed networks are not, suggesting that similarly organized but differentially specialized distributed networks populate association cortex.
Keywords: Broca’s area; Wernicke’s area; distributed association networks; intrinsic functional connectivity; language.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures








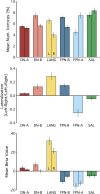
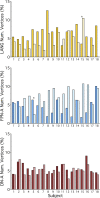
Similar articles
-
Parallel distributed networks dissociate episodic and social functions within the individual.J Neurophysiol. 2020 Mar 1;123(3):1144-1179. doi: 10.1152/jn.00529.2019. Epub 2020 Feb 12. J Neurophysiol. 2020. PMID: 32049593 Free PMC article.
-
Organization of the human cerebral cortex estimated within individuals: networks, global topography, and function.J Neurophysiol. 2024 Jun 1;131(6):1014-1082. doi: 10.1152/jn.00308.2023. Epub 2024 Mar 15. J Neurophysiol. 2024. PMID: 38489238 Free PMC article.
-
Determining hierarchical functional networks from auditory stimuli fMRI.Hum Brain Mapp. 2006 May;27(5):462-70. doi: 10.1002/hbm.20245. Hum Brain Mapp. 2006. PMID: 16568419 Free PMC article.
-
From sensation to cognition.Brain. 1998 Jun;121 ( Pt 6):1013-52. doi: 10.1093/brain/121.6.1013. Brain. 1998. PMID: 9648540 Review.
-
The auditory and association cortex and language evaluation methods.Handb Clin Neurol. 2019;160:465-479. doi: 10.1016/B978-0-444-64032-1.00031-X. Handb Clin Neurol. 2019. PMID: 31277869 Review.
Cited by
-
Functional specialization within the inferior parietal lobes across cognitive domains.Elife. 2021 Mar 2;10:e63591. doi: 10.7554/eLife.63591. Elife. 2021. PMID: 33650486 Free PMC article.
-
Comprehension of computer code relies primarily on domain-general executive brain regions.Elife. 2020 Dec 15;9:e58906. doi: 10.7554/eLife.58906. Elife. 2020. PMID: 33319744 Free PMC article.
-
A two-dimensional space of linguistic representations shared across individuals.bioRxiv [Preprint]. 2025 May 23:2025.05.21.655330. doi: 10.1101/2025.05.21.655330. bioRxiv. 2025. PMID: 40475410 Free PMC article. Preprint.
-
Distinct distributed brain networks dissociate self-generated mental states.bioRxiv [Preprint]. 2025 Feb 27:2025.02.27.640604. doi: 10.1101/2025.02.27.640604. bioRxiv. 2025. PMID: 40060698 Free PMC article. Preprint.
-
Neural populations in the language network differ in the size of their temporal receptive windows.Nat Hum Behav. 2024 Oct;8(10):1924-1942. doi: 10.1038/s41562-024-01944-2. Epub 2024 Aug 26. Nat Hum Behav. 2024. PMID: 39187713
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

