Long-term Clinical and Cost-effectiveness of Early Endovenous Ablation in Venous Ulceration: A Randomized Clinical Trial
- PMID: 32965493
- PMCID: PMC7512122
- DOI: 10.1001/jamasurg.2020.3845
Long-term Clinical and Cost-effectiveness of Early Endovenous Ablation in Venous Ulceration: A Randomized Clinical Trial
Abstract
Importance: One-year outcomes from the Early Venous Reflux Ablation (EVRA) randomized trial showed accelerated venous leg ulcer healing and greater ulcer-free time for participants who are treated with early endovenous ablation of lower extremity superficial reflux.
Objective: To evaluate the clinical and cost-effectiveness of early endovenous ablation of superficial venous reflux in patients with venous leg ulceration.
Design, setting, and participants: Between October 24, 2013, and September 27, 2016, the EVRA randomized clinical trial enrolled 450 participants (450 legs) with venous leg ulceration of less than 6 months' duration and superficial venous reflux. Initially, 6555 patients were assessed for eligibility, and 6105 were excluded for reasons including ulcer duration greater than 6 months, healed ulcer by the time of randomization, deep venous occlusive disease, and insufficient superficial venous reflux to warrant ablation therapy, among others. A total of 426 of 450 participants (94.7%) from the vascular surgery departments of 20 hospitals in the United Kingdom were included in the analysis for ulcer recurrence. Surgeons, participants, and follow-up assessors were not blinded to the treatment group. Data were analyzed from August 11 to November 4, 2019.
Interventions: Patients were randomly assigned to receive compression therapy with early endovenous ablation within 2 weeks of randomization (early intervention, n = 224) or compression with deferred endovenous treatment of superficial venous reflux (deferred intervention, n = 226). Endovenous modality and strategy were left to the preference of the treating clinical team.
Main outcomes and measures: The primary outcome for the extended phase was time to first ulcer recurrence. Secondary outcomes included ulcer recurrence rate and cost-effectiveness.
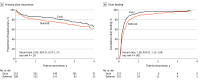
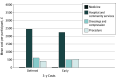
Results: The early-intervention group consisted of 224 participants (mean [SD] age, 67.0 [15.5] years; 127 men [56.7%]; 206 White participants [92%]). The deferred-intervention group consisted of 226 participants (mean [SD] age, 68.9 [14.0] years; 120 men [53.1%]; 208 White participants [92%]). Of the 426 participants whose leg ulcer had healed, 121 (28.4%) experienced at least 1 recurrence during follow-up. There was no clear difference in time to first ulcer recurrence between the 2 groups (hazard ratio, 0.82; 95% CI, 0.57-1.17; P = .28). Ulcers recurred at a lower rate of 0.11 per person-year in the early-intervention group compared with 0.16 per person-year in the deferred-intervention group (incidence rate ratio, 0.658; 95% CI, 0.480-0.898; P = .003). Time to ulcer healing was shorter in the early-intervention group for primary ulcers (hazard ratio, 1.36; 95% CI, 1.12-1.64; P = .002). At 3 years, early intervention was 91.6% likely to be cost-effective at a willingness to pay of £20 000 ($26 283) per quality-adjusted life year and 90.8% likely at a threshold of £35 000 ($45 995) per quality-adjusted life year.
Conclusions and relevance: Early endovenous ablation of superficial venous reflux was highly likely to be cost-effective over a 3-year horizon compared with deferred intervention. Early intervention accelerated the healing of venous leg ulcers and reduced the overall incidence of ulcer recurrence.
Trial registration: ClinicalTrials.gov identifier: ISRCTN02335796.
Conflict of interest statement
Figures
Comment in
-
Importance of Early Saphenous Ablation to Maximize Ulcer-Free Days for Patients With Venous Ulcers.JAMA Surg. 2020 Dec 1;155(12):1121-1122. doi: 10.1001/jamasurg.2020.3846. JAMA Surg. 2020. PMID: 32965481 No abstract available.
Similar articles
-
Early versus deferred endovenous ablation of superficial venous reflux in patients with venous ulceration: the EVRA RCT.Health Technol Assess. 2019 May;23(24):1-96. doi: 10.3310/hta23240. Health Technol Assess. 2019. PMID: 31140402 Free PMC article. Clinical Trial.
-
A Randomized Trial of Early Endovenous Ablation in Venous Ulceration.N Engl J Med. 2018 May 31;378(22):2105-2114. doi: 10.1056/NEJMoa1801214. Epub 2018 Apr 24. N Engl J Med. 2018. PMID: 29688123 Clinical Trial.
-
A randomized trial of early endovenous ablation in venous ulceration: a critical appraisal: Original Article: Gohel MS, Heatly F, Liu X et al. A randomized trial of early endovenous ablation in venous ulceration. N Engl J Med 2018; 378:2105-114.Br J Dermatol. 2019 Jan;180(1):51-55. doi: 10.1111/bjd.17237. Epub 2018 Nov 22. Br J Dermatol. 2019. PMID: 30238444
-
A systematic review on the treatment of nonhealing venous ulcers following successful elimination of superficial venous reflux.J Vasc Surg Venous Lymphat Disord. 2021 Jul;9(4):1071-1076.e1. doi: 10.1016/j.jvsv.2020.12.085. Epub 2021 Feb 26. J Vasc Surg Venous Lymphat Disord. 2021. PMID: 33647527
-
Evidence for varicose vein surgery in venous leg ulceration.Surgeon. 2016 Aug;14(4):219-33. doi: 10.1016/j.surge.2016.03.007. Epub 2016 Apr 16. Surgeon. 2016. PMID: 27095286 Review.
Cited by
-
Chronic Venous Disease of the Lower Extremities: A State-of-the Art Review.J Soc Cardiovasc Angiogr Interv. 2022 Nov 26;2(1):100538. doi: 10.1016/j.jscai.2022.100538. eCollection 2023 Jan-Feb. J Soc Cardiovasc Angiogr Interv. 2022. PMID: 39132527 Free PMC article. Review.
-
Tibial cortex transverse transport facilitating healing in patients with recalcitrant non-diabetic leg ulcers.J Orthop Translat. 2020 Dec 9;27:1-7. doi: 10.1016/j.jot.2020.11.001. eCollection 2021 Mar. J Orthop Translat. 2020. PMID: 33344165 Free PMC article.
-
Comparing methods for handling missing cost and quality of life data in the Early Endovenous Ablation in Venous Ulceration trial.Cost Eff Resour Alloc. 2022 Apr 7;20(1):18. doi: 10.1186/s12962-022-00351-6. Cost Eff Resour Alloc. 2022. PMID: 35392924 Free PMC article.
-
Cost-effectiveness of Compression Therapy With Early Endovenous Ablation in Venous Ulceration for a Medicare Population.JAMA Netw Open. 2022 Dec 1;5(12):e2248152. doi: 10.1001/jamanetworkopen.2022.48152. JAMA Netw Open. 2022. PMID: 36542379 Free PMC article.
-
Patient perceptions and preferences of minimally invasive treatment modalities in varicose veins: a cross-sectional survey.Front Cardiovasc Med. 2024 Apr 25;11:1382764. doi: 10.3389/fcvm.2024.1382764. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38725833 Free PMC article.
References
-
- Berenguer Pérez M, López-Casanova P, Sarabia Lavín R, González de la Torre H, Verdú-Soriano J. Epidemiology of venous leg ulcers in primary health care: incidence and prevalence in a health centre—a time series study (2010-2014). Int Wound J. 2019;16(1):256-265. doi:10.1111/iwj.13026 - DOI - PMC - PubMed
-
- Epstein DM, Gohel MS, Heatley F, et al. ; EVRA trial investigators . Cost-effectiveness analysis of a randomized clinical trial of early versus deferred endovenous ablation of superficial venous reflux in patients with venous ulceration. Br J Surg. 2019;106(5):555-562. doi:10.1002/bjs.11082 - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

