Safety and Efficacy of VP-102, a Proprietary, Drug-Device Combination Product Containing Cantharidin, 0.7% (w/v), in Children and Adults With Molluscum Contagiosum: Two Phase 3 Randomized Clinical Trials
- PMID: 32965495
- PMCID: PMC7512131
- DOI: 10.1001/jamadermatol.2020.3238
Safety and Efficacy of VP-102, a Proprietary, Drug-Device Combination Product Containing Cantharidin, 0.7% (w/v), in Children and Adults With Molluscum Contagiosum: Two Phase 3 Randomized Clinical Trials
Abstract
Importance: Molluscum contagiosum (MC) is a common viral skin infection that primarily affects children. Cantharidin, a topical vesicant, has a long history of use for MC in compounded formulations, but the safety and efficacy of doses, regimens, and application methods have not been demonstrated in large-scale trials.
Objective: To determine the safety and efficacy of VP-102, a drug-device combination containing cantharidin, 0.7% (w/v), compared with vehicle in individuals with MC.
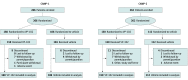
Design, setting, and participants: Two phase 3, randomized, double-blind, vehicle-controlled trials of identical design (Cantharidin Application in Molluscum Patients [CAMP-1 and CAMP-2]) were conducted in 31 centers across the US. A total of 528 individuals aged 2 years or older with MC participated. CAMP-1 was conducted from March 21 to November 26, 2018, and CAMP-2 was conducted from February 14 to September 26, 2018.
Interventions: Participants were randomized (3:2) to topical application of VP-102 or vehicle to all treatable lesions every 21 days until complete lesion clearance or up to 4 treatments.
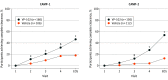
Main outcomes and measures: The primary efficacy outcome was the proportion of VP-102-treated participants achieving complete clearance of all MC lesions (baseline and new) compared with those who received the vehicle at the end-of-study visit on day 84. Intent-to-treat analysis was conducted for the efficacy population. Secondary efficacy outcomes included the proportion of participants achieving complete clearance of lesions at days 21, 42, and 63. Safety outcomes included assessment of adverse events, including expected local skin reactions.
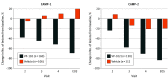
Results: Of the 528 participants enrolled, 527 received treatment (CAMP-1, n = 265; CAMP-2, n = 262). A total of 267 of 527 participants (50.7%) were male; mean (SD) ages for CAMP-1 and CAMP-2 were 7.5 (5.3) years and 7.4 (8.0) years for the VP-102 groups and 6.3 (4.7) years and 7.3 (6.7) years for the vehicle groups. Treatment with VP-102 demonstrated superior efficacy to vehicle in the percentage of participants with complete clearance of MC lesions at the end of the study visit for CAMP-1 (VP-102: 46.3% vs vehicle: 17.9%; P < .001) and CAMP-2 (VP-102: 54.0% vs vehicle: 13.4%; P < .001). Adverse events were observed in 99% (CAMP-1) and 95% (CAMP-2) of VP-102-treated participants and 73% (CAMP-1) and 66% (CAMP-2) of vehicle-treated participants. The most common adverse events included application site vesicles, pain, pruritus, erythema, and scab. Most adverse events were mild or moderate in severity.
Conclusions and relevance: In the 2 phase 3 trials reported herein, VP-102 was statistically significantly superior to vehicle in achieving complete clearance of MC lesions at the end of the study visit in both trials, with adverse events that were generally mild to moderate and confined to application sites. These findings show that VP-102 is potentially an effective and safe treatment for MC, a common skin condition with no US Food and Drug Administration-approved treatments.
Trial registrations: ClinicalTrials.gov Identifiers: NCT03377790 and NCT03377803.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

