Efficacy of Biologics Targeting Tumour Necrosis Factor-alpha, Interleukin-17 -12/23, -23 and Small Molecules Targeting JAK and PDE4 in the Treatment of Nail Psoriasis: A Network Meta-analysis
- PMID: 32965504
- PMCID: PMC9309877
- DOI: 10.2340/00015555-3640
Efficacy of Biologics Targeting Tumour Necrosis Factor-alpha, Interleukin-17 -12/23, -23 and Small Molecules Targeting JAK and PDE4 in the Treatment of Nail Psoriasis: A Network Meta-analysis
Abstract
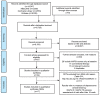
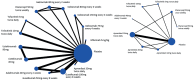
The comparative efficacy of registered anti-psoriatic biologics and small molecules in treating nail symptoms has not been systematically evaluated. The aim of this study was to perform a network meta-analysis to determine the efficacy of biologics and small mole-cules in nail psoriasis. A Bayesian network meta- analysis of 17 randomized clinical trials (a total of 6,053 nail psoriatic patients) was performed, comparing the short-term (week 10-16) efficacy of biologics and small molecules in the treatment of nail psoriasis. All active treatments were found to be superior to place-bo. Ixekizumab 80 mg every 4 weeks (Nail Psoriasis Severity Index (NAPSI) % improvement, Surface Under the Cumulative Ranking (SUCRA)=0.92) and etanercept 50 mg twice weekly (probability of achiev-ing NAPSI 50, SUCRA=0.82) proved the best short-term treatment options. However, efficacy end-points in psoriasis trials were not optimized for nail assessment, and outcome parameters were highly heterogeneous, limiting comparability. In conclusion, outcome parameters and efficacy endpoints of nail psoriasis trials should be standardized.
Keywords: Nail Psoriasis Severity Index; biologics; efficacy; network meta-analysis; nail psoriasis.
Figures
Similar articles
-
Network meta-analysis comparing the efficacy of biologic treatments for achieving complete resolution of nail psoriasis.J Dermatolog Treat. 2022 May;33(3):1652-1660. doi: 10.1080/09546634.2021.1892024. Epub 2021 Mar 1. J Dermatolog Treat. 2022. PMID: 33641593
-
Ixekizumab Is Effective in Subjects With Moderate to Severe Plaque Psoriasis With Significant Nail Involvement: Results From UNCOVER 3.J Drugs Dermatol. 2016 Aug 1;15(8):958-61. J Drugs Dermatol. 2016. PMID: 27537996 Clinical Trial.
-
Network meta-analyses comparing the efficacy of biologic treatments for achieving complete resolution of nail psoriasis at 24-28 and 48-52 weeks.J Dermatolog Treat. 2023 Dec;34(1):2263108. doi: 10.1080/09546634.2023.2263108. Epub 2023 Oct 2. J Dermatolog Treat. 2023. PMID: 37781881
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2023 Jul 12;7(7):CD011535. doi: 10.1002/14651858.CD011535.pub6. Cochrane Database Syst Rev. 2023. PMID: 37436070 Free PMC article. Review.
-
Nail psoriasis and biologics.J Cutan Med Surg. 2009 Jan-Feb;13(1):1-5. doi: 10.2310/7750.2008.08012. J Cutan Med Surg. 2009. PMID: 19298765 Review.
Cited by
-
A Comprehensive Review of Ixekizumab Efficacy in Nail Psoriasis from Clinical Trials for Moderate-to-Severe Psoriasis and Psoriatic Arthritis.Rheumatol Ther. 2023 Oct;10(5):1127-1146. doi: 10.1007/s40744-023-00553-1. Epub 2023 Jul 3. Rheumatol Ther. 2023. PMID: 37400681 Free PMC article. Review.
-
Narrative Review of the Emerging Therapeutic Role of Brodalumab in Difficult-to-Treat Psoriasis.Dermatol Ther (Heidelb). 2022 Jun;12(6):1289-1302. doi: 10.1007/s13555-022-00746-6. Epub 2022 Jun 7. Dermatol Ther (Heidelb). 2022. PMID: 35672564 Free PMC article. Review.
-
Real-World Evidence for Ixekizumab in the Treatment of Psoriasis, Psoriatic Arthritis, and Axial Spondyloarthritis: Systematic Literature Review 2022-2023.Adv Ther. 2025 Jul 17. doi: 10.1007/s12325-025-03258-9. Online ahead of print. Adv Ther. 2025. PMID: 40676380 Review.
-
Efficacy and Safety of Ixekizumab Versus Adalimumab in Biologic-naïve Patients With Active Psoriatic Arthritis and Moderate-to-severe Psoriasis: 52-week Results From the Randomized SPIRIT-H2H Trial.Dermatol Pract Concept. 2022 Apr 1;12(2):e2022104. doi: 10.5826/dpc.1202a104. eCollection 2022 May. Dermatol Pract Concept. 2022. PMID: 35646453 Free PMC article.
-
Association between IL-17F, IL-17RA Gene Polymorphisms and Response to Biological Drugs in Psoriasis and Beyond.Genes (Basel). 2023 May 22;14(5):1123. doi: 10.3390/genes14051123. Genes (Basel). 2023. PMID: 37239484 Free PMC article.
References
-
- Blome C, Costanzo A, Dauden E, Ferrandiz C, Girolomoni G, Gniadecki R, et al. . Patient-relevant needs and treatment goals in nail psoriasis. Qual Life Res 2016; 25: 1179–1188. - PubMed
-
- Augustin M, Reich K, Blome C, Schäfer I, Laass A, Radtke MA. Nail psoriasis in Germany: epidemiology and burden of disease. Br J Dermatol 2010; 163: 580–585. - PubMed
-
- Baran R. The burden of nail psoriasis: an introduction. Dermatology 2010; 221: 1–5. - PubMed
-
- McGonagle D, Tan AL, Benjamin M. The nail as a musculoskeletal appendage – implications for an improved understanding of the link between psoriasis and arthritis. Dermatology 2009; 218: 97–102. - PubMed
-
- Radtke MA, Beikert FC, Augustin M. Nail psoriasis – a treatment challenge. J Dtsch Dermatol Ges 2013; 11: 203–219; quiz 220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

