Efficacy and Safety of CT-P13 in Inflammatory Bowel Disease after Switching from Originator Infliximab: Exploratory Analyses from the NOR-SWITCH Main and Extension Trials
- PMID: 32965617
- PMCID: PMC7519917
- DOI: 10.1007/s40259-020-00438-7
Efficacy and Safety of CT-P13 in Inflammatory Bowel Disease after Switching from Originator Infliximab: Exploratory Analyses from the NOR-SWITCH Main and Extension Trials
Abstract
Background: The NOR-SWITCH main and extension trials demonstrated that switching from originator to biosimilar infliximab (CT-P13) is efficacious and safe across six diseases. However, a subgroup analysis of Crohn's disease (CD) in the main trial displayed a close to significant difference favouring originator infliximab, and more scientific data have therefore been requested.
Objective: The aim was to assess treatment efficacy, safety, and immunogenicity in an explorative subgroup analysis in CD and ulcerative colitis (UC) in the NOR-SWITCH trials.
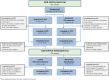
Patients and methods: The 52-week, randomised, non-inferiority, double-blind, multicentre, phase 4 NOR-SWITCH study was followed by a 26-week open extension trial where all patients received treatment with CT-P13. Treatment efficacy, safety, and immunogenicity in CD and UC were assessed throughout the 78-week study period.
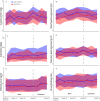
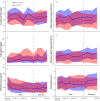
Results: The main and extension trials included 155 and 93 patients with CD and 93 and 80 patients with UC, respectively. Demographic and baseline characteristics were comparable in both treatment arms within patient groups. There were no differences in the main and extension trials regarding changes in activity indices, C-reactive protein, faecal calprotectin, patient's and physician's global assessment of disease activity and patient-reported outcome measures in CD and UC. Moreover, comparable results were also demonstrated for trough serum levels, presence of anti-drug antibodies, and reported adverse events.
Conclusion: Efficacy, safety, and immunogenicity of both the originator and biosimilar infliximab were comparable in CD and UC in the NOR-SWITCH main and extension trials. These explorative subgroup analyses confirm that there are no significant concerns related to switching from originator infliximab to CT-P13 in CD and UC.
Trial registration: ClinicalTrials.gov, number NCT02148640.
Conflict of interest statement
Figures
Similar articles
-
Serum concentrations after switching from originator infliximab to the biosimilar CT-P13 in patients with quiescent inflammatory bowel disease (SECURE): an open-label, multicentre, phase 4 non-inferiority trial.Lancet Gastroenterol Hepatol. 2018 Jun;3(6):404-412. doi: 10.1016/S2468-1253(18)30082-7. Epub 2018 Mar 30. Lancet Gastroenterol Hepatol. 2018. PMID: 29606564 Clinical Trial.
-
Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial.Lancet. 2017 Jun 10;389(10086):2304-2316. doi: 10.1016/S0140-6736(17)30068-5. Epub 2017 May 11. Lancet. 2017. PMID: 28502609 Clinical Trial.
-
Efficacy and Safety of Elective Switching from Intravenous to Subcutaneous Infliximab [CT-P13]: A Multicentre Cohort Study.J Crohns Colitis. 2022 Sep 8;16(9):1436-1446. doi: 10.1093/ecco-jcc/jjac053. J Crohns Colitis. 2022. PMID: 35390141 Free PMC article.
-
Evolution of infliximab biosimilar in inflammatory bowel disease: from intravenous to subcutaneous CT-P13.Expert Opin Biol Ther. 2021 Jan;21(1):37-46. doi: 10.1080/14712598.2020.1811849. Epub 2020 Aug 31. Expert Opin Biol Ther. 2021. PMID: 32799561 Review.
-
Systematic Review: Non-medical Switching of Infliximab to CT-P13 in Inflammatory Bowel Disease.Dig Dis Sci. 2020 Aug;65(8):2354-2372. doi: 10.1007/s10620-019-06036-0. Epub 2020 Jan 22. Dig Dis Sci. 2020. PMID: 31970610 Free PMC article.
Cited by
-
Immunogenicity of Therapeutic Antibodies Used for Inflammatory Bowel Disease: Treatment and Clinical Considerations.Drugs. 2025 Jan;85(1):67-85. doi: 10.1007/s40265-024-02115-3. Epub 2024 Nov 13. Drugs. 2025. PMID: 39532820 Review.
-
Infliximab for maintenance of medically-induced remission in Crohn's disease.Cochrane Database Syst Rev. 2024 Feb 19;2(2):CD012609. doi: 10.1002/14651858.CD012609.pub2. Cochrane Database Syst Rev. 2024. PMID: 38372447 Free PMC article.
-
An Update on Anti-TNF Biosimilar Switching-Real-World Clinical Effectiveness and Safety.J Can Assoc Gastroenterol. 2023 Oct 25;7(1):30-45. doi: 10.1093/jcag/gwad027. eCollection 2024 Feb. J Can Assoc Gastroenterol. 2023. PMID: 38314175 Free PMC article.
-
Reverse switching from the biosimilar SB2 to the originator infliximab in previously switched patients with inflammatory bowel diseases: results of a prospective long-term cohort study.Therap Adv Gastroenterol. 2024 Nov 30;17:17562848241301887. doi: 10.1177/17562848241301887. eCollection 2024. Therap Adv Gastroenterol. 2024. PMID: 39619829 Free PMC article.
-
Evaluating the Efficacy of Infliximab in Inflammatory Bowel Disease: A Systematic Review of the Literature.Cureus. 2024 Aug 1;16(8):e65971. doi: 10.7759/cureus.65971. eCollection 2024 Aug. Cureus. 2024. PMID: 39221404 Free PMC article. Review.
References
-
- Park W, Hrycaj P, Jeka S, Kovalenko V, Lysenko G, Miranda P, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72(10):1605–1612. doi: 10.1136/annrheumdis-2012-203091. - DOI - PMC - PubMed
-
- Yoo DH, Hrycaj P, Miranda P, Ramiterre E, Piotrowski M, Shevchuk S, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72(10):1613–1620. doi: 10.1136/annrheumdis-2012-203090. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

