Serial Testing for SARS-CoV-2 and Virus Whole Genome Sequencing Inform Infection Risk at Two Skilled Nursing Facilities with COVID-19 Outbreaks - Minnesota, April-June 2020
- PMID: 32966272
- PMCID: PMC7498172
- DOI: 10.15585/mmwr.mm6937a3
Serial Testing for SARS-CoV-2 and Virus Whole Genome Sequencing Inform Infection Risk at Two Skilled Nursing Facilities with COVID-19 Outbreaks - Minnesota, April-June 2020
Abstract
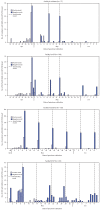
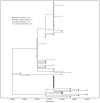
SARS-CoV-2, the virus that causes coronavirus disease 2019 (COVID-19), can spread rapidly in high-risk congregate settings such as skilled nursing facilities (SNFs) (1). In Minnesota, SNF-associated cases accounted for 3,950 (8%) of 48,711 COVID-19 cases reported through July 21, 2020; 35% of SNF-associated cases involved health care personnel (HCP*), including six deaths. Facility-wide, serial testing in SNFs has been used to identify residents with asymptomatic and presymptomatic SARS-CoV-2 infection to inform mitigation efforts, including cohorting of residents with positive test results and exclusion of infected HCP from the workplace (2,3). During April-June 2020, the Minnesota Department of Health (MDH), with CDC assistance, conducted weekly serial testing at two SNFs experiencing COVID-19 outbreaks. Among 259 tested residents, and 341 tested HCP, 64% and 33%, respectively, had positive reverse transcription-polymerase chain reaction (RT-PCR) SARS-CoV-2 test results. Continued SARS-CoV-2 transmission was potentially facilitated by lapses in infection prevention and control (IPC) practices, up to 12-day delays in receiving HCP test results (53%) at one facility, and incomplete HCP participation (71%). Genetic sequencing demonstrated that SARS-CoV-2 viral genomes from HCP and resident specimens were clustered by facility, suggesting facility-based transmission. Residents and HCP working in SNFs are at risk for infection with SARS-CoV-2. As part of comprehensive COVID-19 preparation and response, including early identification of cases, SNFs should conduct serial testing of residents and HCP, maximize HCP testing participation, ensure availability of personal protective equipment (PPE), and enhance IPC practices† (4-5).
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
Similar articles
-
Initial and Repeated Point Prevalence Surveys to Inform SARS-CoV-2 Infection Prevention in 26 Skilled Nursing Facilities - Detroit, Michigan, March-May 2020.MMWR Morb Mortal Wkly Rep. 2020 Jul 10;69(27):882-886. doi: 10.15585/mmwr.mm6927e1. MMWR Morb Mortal Wkly Rep. 2020. PMID: 32644985 Free PMC article.
-
Asymptomatic and Presymptomatic SARS-CoV-2 Infections in Residents of a Long-Term Care Skilled Nursing Facility - King County, Washington, March 2020.MMWR Morb Mortal Wkly Rep. 2020 Apr 3;69(13):377-381. doi: 10.15585/mmwr.mm6913e1. MMWR Morb Mortal Wkly Rep. 2020. PMID: 32240128 Free PMC article.
-
Universal and Serial Laboratory Testing for SARS-CoV-2 at a Long-Term Care Skilled Nursing Facility for Veterans - Los Angeles, California, 2020.MMWR Morb Mortal Wkly Rep. 2020 May 29;69(21):651-655. doi: 10.15585/mmwr.mm6921e1. MMWR Morb Mortal Wkly Rep. 2020. PMID: 32463809 Free PMC article.
-
How should a positive PCR test result for COVID-19 in an asymptomatic individual be interpreted and managed?Med Mal Infect. 2020 Nov;50(8):633-638. doi: 10.1016/j.medmal.2020.09.014. Epub 2020 Oct 3. Med Mal Infect. 2020. PMID: 33022291 Free PMC article. Review. No abstract available.
-
Diagnostic and methodological evaluation of studies on the urinary shedding of SARS-CoV-2, compared to stool and serum: A systematic review and meta-analysis.Cell Mol Biol (Noisy-le-grand). 2020 Sep 30;66(6):148-156. Cell Mol Biol (Noisy-le-grand). 2020. PMID: 33040802
Cited by
-
SARS-CoV-2 in Nursing Homes after 3 Months of Serial, Facilitywide Point Prevalence Testing, Connecticut, USA.Emerg Infect Dis. 2021 May;27(5):1288-1295. doi: 10.3201/eid2705.204936. Emerg Infect Dis. 2021. PMID: 33900171 Free PMC article. Review.
-
Surveillance for Unexplained Deaths of Possible Infectious Etiologies During the COVID-19 Pandemic-Minnesota, 2020-2021.Public Health Rep. 2024 May-Jun;139(3):325-332. doi: 10.1177/00333549231218283. Epub 2024 Jan 11. Public Health Rep. 2024. PMID: 38205808 Free PMC article.
-
Transmission Dynamics of Severe Acute Respiratory Syndrome Coronavirus 2 in High-Density Settings, Minnesota, USA, March-June 2020.Emerg Infect Dis. 2021 Aug;27(8):2052-2063. doi: 10.3201/eid2708.204838. Epub 2021 Jun 17. Emerg Infect Dis. 2021. PMID: 34138695 Free PMC article.
-
Analysis of the Transmission of SARS-CoV-2 Delta VOC in Yantai, China, August 2021.Front Med (Lausanne). 2022 May 30;9:842719. doi: 10.3389/fmed.2022.842719. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35707526 Free PMC article.
-
Field and Molecular Epidemiology: How Viral Sequencing Changed Transmission Inferences in the First Portuguese SARS-CoV-2 Infection Cluster.Viruses. 2021 Jun 10;13(6):1116. doi: 10.3390/v13061116. Viruses. 2021. PMID: 34200621 Free PMC article.
References
-
- CDC. Coronavirus disease 2019 (COVID-19): testing guidance for nursing homes. Atlanta, GA: US Department of Health and Human Services, CDC; 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/nursing-homes-testing.html
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

