A 3D brain unit model to further improve prediction of local drug distribution within the brain
- PMID: 32966285
- PMCID: PMC7511021
- DOI: 10.1371/journal.pone.0238397
A 3D brain unit model to further improve prediction of local drug distribution within the brain
Abstract
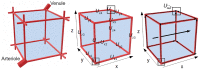
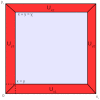
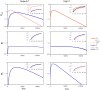
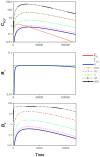
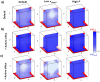
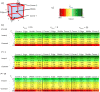
The development of drugs targeting the brain still faces a high failure rate. One of the reasons is a lack of quantitative understanding of the complex processes that govern the pharmacokinetics (PK) of a drug within the brain. While a number of models on drug distribution into and within the brain is available, none of these addresses the combination of factors that affect local drug concentrations in brain extracellular fluid (brain ECF). Here, we develop a 3D brain unit model, which builds on our previous proof-of-concept 2D brain unit model, to understand the factors that govern local unbound and bound drug PK within the brain. The 3D brain unit is a cube, in which the brain capillaries surround the brain ECF. Drug concentration-time profiles are described in both a blood-plasma-domain and a brain-ECF-domain by a set of differential equations. The model includes descriptions of blood plasma PK, transport through the blood-brain barrier (BBB), by passive transport via paracellular and transcellular routes, and by active transport, and drug binding kinetics. The impact of all these factors on ultimate local brain ECF unbound and bound drug concentrations is assessed. In this article we show that all the above mentioned factors affect brain ECF PK in an interdependent manner. This indicates that for a quantitative understanding of local drug concentrations within the brain ECF, interdependencies of all transport and binding processes should be understood. To that end, the 3D brain unit model is an excellent tool, and can be used to build a larger network of 3D brain units, in which the properties for each unit can be defined independently to reflect local differences in characteristics of the brain.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Summerfield SG, Stevens AJ, Cutler L, del Carmen Osuna M, Hammond B, Tang SP, et al. Improving the in Vitro Prediction of in Vivo Central Nervous System Penetration: Integrating Permeability, P-glycoprotein Efflux, and Free Fractions in Blood and Brain. The Journal of Pharmacology and Experimental Therapeutics. 2006;316(3):1282–1290. 10.1124/jpet.105.092916 - DOI - PubMed
-
- van Bree JB, de Boer AG, Danhof M, Ginsel LA, Breimer DD. Characterization of an “in vitro” blood-brain barrier: effects of molecular size and lipophilicity on cerebrovascular endothelial transport rates of drugs. Journal of Pharmacology and Experimental Therapeutics. 1988;247(3):1233–1239. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

