Aberrant BMP2 Signaling in Patients Diagnosed with Osteoporosis
- PMID: 32967078
- PMCID: PMC7555210
- DOI: 10.3390/ijms21186909
Aberrant BMP2 Signaling in Patients Diagnosed with Osteoporosis
Abstract
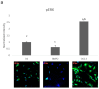
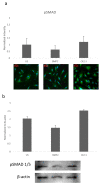
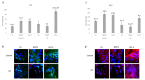
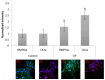
The most common bone disease in humans is osteoporosis (OP). Current therapeutics targeting OP have several negative side effects. Bone morphogenetic protein 2 (BMP2) is a potent growth factor that is known to activate both osteoblasts and osteoclasts. It completes these actions through both SMAD-dependent and SMAD-independent signaling. A novel interaction between the BMP type Ia receptor (BMPRIa) and casein kinase II (CK2) was discovered, and several CK2 phosphorylation sites were identified. A corresponding blocking peptide (named CK2.3) was designed to further elucidate the phosphorylation site's function. Previously, CK2.3 demonstrated an increased osteoblast activity and decreased osteoclast activity in a variety of animal models, cell lines, and isolated human osteoblasts. It is hypothesized that CK2.3 completes these actions through the BMP signaling pathway. Furthermore, it was recently discovered that BMP2 did not elicit an osteogenic response in osteoblasts from patients diagnosed with OP, while CK2.3 did. In this study, we explore where in the BMP pathway the signaling disparity or defect lies in those diagnosed with OP. We found that osteoblasts isolated from patients diagnosed with OP did not activate SMAD or ERK signaling after BMP2 stimulation. When OP osteoblasts were stimulated with BMP2, both BMPRIa and CK2 expression significantly decreased. This indicates a major disparity within the BMP signaling pathway in patients diagnosed with osteoporosis.
Keywords: BMP2; BMPRIa; CK2; CK2.3; osteoporosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


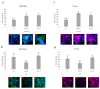
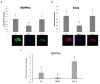
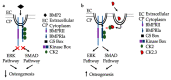
References
-
- Facts and Statistics | International Osteoporosis Foundation. [(accessed on 23 March 2020)]; Available online: https://www.iofbonehealth.org/facts-statistics.
-
- Office of the Surgeon General (US) Bone Health and Osteoporosis. [(accessed on 23 March 2020)];2004 Available online: https://www.ncbi.nlm.nih.gov/books/NBK45513/ - PubMed
-
- PubMed Homepage. [(accessed on 2 March 2012)]; Available online: http://www.ncbi.nlm.nih.gov/pubmed/
-
- Chavassieux P., Chapurlat R., Portero-Muzy N., Roux J.-P., Garcia P., Brown J.P., Libanati C., Boyce R.W., Wang A., Grauer A. Bone-Forming and Antiresorptive Effects of Romosozumab in Postmenopausal Women with Osteoporosis: Bone Histomorphometry and Microcomputed Tomography Analysis after 2 and 12 Months of Treatment. J. Bone Miner. Res. 2019;34:1597–1608. doi: 10.1002/jbmr.3735. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

