Figainin 1, a Novel Amphibian Skin Peptide with Antimicrobial and Antiproliferative Properties
- PMID: 32967114
- PMCID: PMC7559428
- DOI: 10.3390/antibiotics9090625
Figainin 1, a Novel Amphibian Skin Peptide with Antimicrobial and Antiproliferative Properties
Abstract
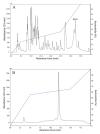
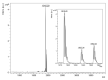
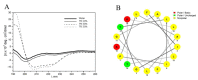
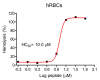
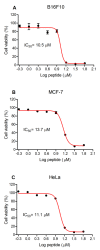
Amphibian skin secretions are abundant in bioactive compounds, especially antimicrobial peptides. These molecules are generally cationic and rich in hydrophobic amino acids, have an amphipathic structure and adopt an α-helical conformation when in contact with microorganisms membranes. In this work, we purified and characterized Figainin 1, a novel antimicrobial and antiproliferative peptide from the cutaneous secretion of the frog Boana raniceps. Figainin 1 is a cationic peptide with eighteen amino acid residues-rich in leucine and isoleucine, with an amidated C-terminus-and adopts an α-helical conformation in the presence of trifluoroethanol (TFE). It displayed activity against Gram-negative and especially Gram-positive bacteria, with MIC values ranging from 2 to 16 µM, and showed an IC50 value of 15.9 µM against epimastigote forms of T. cruzi; however, Figanin 1 did not show activity against Candida species. This peptide also showed cytolytic effects against human erythrocytes with an HC50 of 10 µM, in addition to antiproliferative activity against cancer cells and murine fibroblasts, with IC50 values ranging from 10.5 to 13.7 µM. Despite its adverse effects on noncancerous cells, Figainin 1 exhibits interesting properties for the development of new anticancer agents and anti-infective drugs against pathogenic microorganisms.
Keywords: Boana raniceps; amphibian; antimicrobial peptide; cytolytic peptide; hemolysis; skin secretion; structural analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

