Microglia and Macrophages in the Pathological Central and Peripheral Nervous Systems
- PMID: 32967118
- PMCID: PMC7563796
- DOI: 10.3390/cells9092132
Microglia and Macrophages in the Pathological Central and Peripheral Nervous Systems
Abstract
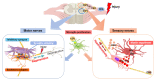
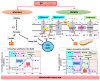
Microglia, the immunocompetent cells in the central nervous system (CNS), have long been studied as pathologically deteriorating players in various CNS diseases. However, microglia exert ameliorating neuroprotective effects, which prompted us to reconsider their roles in CNS and peripheral nervous system (PNS) pathophysiology. Moreover, recent findings showed that microglia play critical roles even in the healthy CNS. The microglial functions that normally contribute to the maintenance of homeostasis in the CNS are modified by other cells, such as astrocytes and infiltrated myeloid cells; thus, the microglial actions on neurons are extremely complex. For a deeper understanding of the pathophysiology of various diseases, including those of the PNS, it is important to understand microglial functioning. In this review, we discuss both the favorable and unfavorable roles of microglia in neuronal survival in various CNS and PNS disorders. We also discuss the roles of blood-borne macrophages in the pathogenesis of CNS and PNS injuries because they cooperatively modify the pathological processes of resident microglia. Finally, metabolic changes in glycolysis and oxidative phosphorylation, with special reference to the pro-/anti-inflammatory activation of microglia, are intensively addressed, because they are profoundly correlated with the generation of reactive oxygen species and changes in pro-/anti-inflammatory phenotypes.
Keywords: NG2; brain infarction; carbon monoxide poisoning; macrophage; peripheral nerve injury; traumatic brain injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

