Boronic Acids and Their Derivatives in Medicinal Chemistry: Synthesis and Biological Applications
- PMID: 32967170
- PMCID: PMC7571202
- DOI: 10.3390/molecules25184323
Boronic Acids and Their Derivatives in Medicinal Chemistry: Synthesis and Biological Applications
Abstract
Boron containing compounds have not been widely studied in Medicinal Chemistry, mainly due to the idea that this group could confer some toxicity. Nowadays, this concept has been demystified and, especially after the discovery of the drug bortezomib, the interest for these compounds, mainly boronic acids, has been growing. In this review, several activities of boronic acids, such as anticancer, antibacterial, antiviral activity, and even their application as sensors and delivery systems are addressed. The synthetic processes used to obtain these active compounds are also referred. Noteworthy, the molecular modification by the introduction of boronic acid group to bioactive molecules has shown to modify selectivity, physicochemical, and pharmacokinetic characteristics, with the improvement of the already existing activities. Besides, the preparation of compounds with this chemical group is relatively simple and well known. Taking into consideration these findings, this review reinforces the relevance of extending the studies with boronic acids in Medicinal Chemistry, in order to obtain new promising drugs shortly.
Keywords: biological applications; boron-containing compounds; boronic acids; synthesis of boronic acid derivatives.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
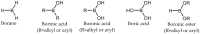

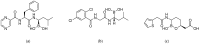

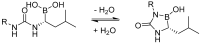
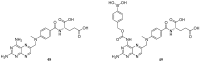
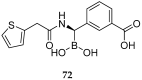
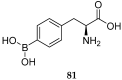
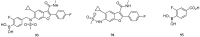

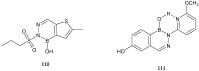
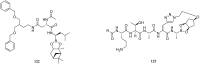
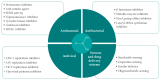
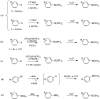
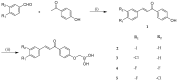
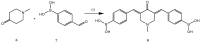
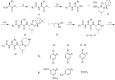
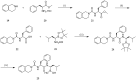
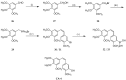
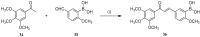
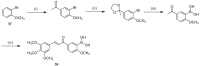

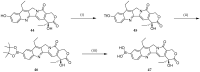
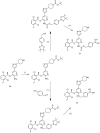
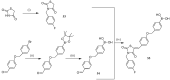


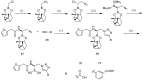
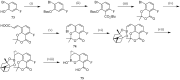
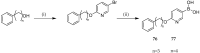



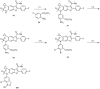
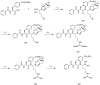
References
-
- Frankland E., Duppa B.F. Vorläufige Notiz über Boräthyl. Justus Liebigs Ann. Chem. 1860;115:319–322. doi: 10.1002/jlac.18601150324. - DOI
-
- Hall D.G., editor. Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine. Wiley-VCH; Weinheim, Germany: 2005. Structure, Properties, and Preparation of Boronic Acid Derivatives. Overview of Their Reactions and Applications; pp. 1–99.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

