Skin Damages-Structure Activity Relationship of Benzimidazole Derivatives Bearing a 5-Membered Ring System
- PMID: 32967192
- PMCID: PMC7570844
- DOI: 10.3390/molecules25184324
Skin Damages-Structure Activity Relationship of Benzimidazole Derivatives Bearing a 5-Membered Ring System
Abstract
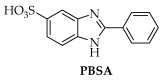
In the search for scaffolds for multifunctional compounds we investigated the structure activity relationship of a class of benzimidazole derivatives bearing 5-membered ring. The newly synthesized and the already known compounds were divided into three classes that present different substituent at 5 position of the benzimidazole ring (-H, -COOH or -SO3H) and different heterocycle at position 2 (thiophene, furan or pyrrole). All the derivatives were synthesized and tested to determine their photoprotective profile against UV rays, in vitro antioxidant capacity against different radicals (DPPH and FRAP test), antifungal inhibitory activity (dermatophytes and Candida albicans), antiviral and antiproliferative activity. A Structure-Activity Relationship study indicated compound 10, bearing a pyrrole heterocycle on the benzimidazole ring, as the best multifunctional derivative of the series and as potential candidate for the development of drugs especially in case of melanoma.
Keywords: UV-filter; benzimidazole; melanoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


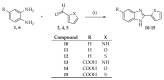

Similar articles
-
A Multitarget Approach toward the Development of 8-Substituted Purines for Photoprotection and Prevention of UV-Related Damage.ChemMedChem. 2017 May 22;12(10):760-769. doi: 10.1002/cmdc.201700137. Epub 2017 May 3. ChemMedChem. 2017. PMID: 28417557
-
Synthesis and evaluation of antioxidant and antiproliferative activity of 2-arylbenzimidazoles.Bioorg Chem. 2020 Jan;94:103396. doi: 10.1016/j.bioorg.2019.103396. Epub 2019 Oct 24. Bioorg Chem. 2020. PMID: 31677860
-
Design, synthesis and biological evaluation of novel hydroxy-phenyl-1H-benzimidazoles as radical scavengers and UV-protective agents.J Enzyme Inhib Med Chem. 2017 Dec;32(1):527-537. doi: 10.1080/14756366.2016.1265523. J Enzyme Inhib Med Chem. 2017. PMID: 28114824 Free PMC article.
-
Benzimidazole Scaffold as Anticancer Agent: Synthetic Approaches and Structure-Activity Relationship.Arch Pharm (Weinheim). 2017 Jun;350(6). doi: 10.1002/ardp.201700040. Epub 2017 May 22. Arch Pharm (Weinheim). 2017. PMID: 28544162 Review.
-
Some characteristics of activity of potential chemotherapeutics--benzimidazole derivatives.Adv Med Sci. 2015 Mar;60(1):125-32. doi: 10.1016/j.advms.2015.01.004. Epub 2015 Jan 21. Adv Med Sci. 2015. PMID: 25725479 Review.
Cited by
-
Nitrogen-Containing Heterocycles as Significant Molecular Scaffolds for Medicinal and Other Applications.Molecules. 2021 Jul 30;26(15):4617. doi: 10.3390/molecules26154617. Molecules. 2021. PMID: 34361770 Free PMC article.
-
UV Filters: Challenges and Prospects.Pharmaceuticals (Basel). 2022 Feb 22;15(3):263. doi: 10.3390/ph15030263. Pharmaceuticals (Basel). 2022. PMID: 35337062 Free PMC article. Review.
-
Design, Synthesis and Evaluation of New Multifunctional Benzothiazoles as Photoprotective, Antioxidant and Antiproliferative Agents.Molecules. 2022 Dec 29;28(1):287. doi: 10.3390/molecules28010287. Molecules. 2022. PMID: 36615480 Free PMC article.
-
Benzothiazole Derivatives as Multifunctional Antioxidant Agents for Skin Damage: Structure-Activity Relationship of a Scaffold Bearing a Five-Membered Ring System.Antioxidants (Basel). 2022 Feb 17;11(2):407. doi: 10.3390/antiox11020407. Antioxidants (Basel). 2022. PMID: 35204288 Free PMC article.
-
Eco-Friendly Synthesis of 1H-benzo[d]imidazole Derivatives by ZnO NPs Characterization, DFT Studies, Antioxidant and Insilico Studies.Pharmaceuticals (Basel). 2023 Jul 6;16(7):969. doi: 10.3390/ph16070969. Pharmaceuticals (Basel). 2023. PMID: 37513881 Free PMC article.
References
-
- Saewan N., Jimtaisong A. Photoprotection of natural flavonoids. J. Appl. Pharm. Sci. 2013;3:129–141.
-
- Laleh Farajia S.S. Synthesis of novel benzimidazole and benzothiazole derivatives bearing a 1,2,3-triazole ring system and their acetylcholinesterase inhibitory activity. J. Chem. Res. 2017;41:30–35. doi: 10.3184/174751917X14836231670980. - DOI
-
- Joule J.A., Mills K. Heterocyclic Chemistry. 5th ed. Wiley India Pvt. Ltd.; New Delhi, India: 2010. pp. 503–506.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

