Impact of Exercise on Immunometabolism in Multiple Sclerosis
- PMID: 32967206
- PMCID: PMC7564219
- DOI: 10.3390/jcm9093038
Impact of Exercise on Immunometabolism in Multiple Sclerosis
Abstract
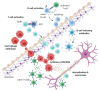
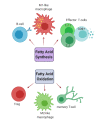
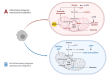
Multiple Sclerosis (MS) is a chronic, autoimmune condition characterized by demyelinating lesions and axonal degradation. Even though the cause of MS is heterogeneous, it is known that peripheral immune invasion in the central nervous system (CNS) drives pathology at least in the most common form of MS, relapse-remitting MS (RRMS). The more progressive forms' mechanisms of action remain more elusive yet an innate immune dysfunction combined with neurodegeneration are likely drivers. Recently, increasing studies have focused on the influence of metabolism in regulating immune cell function. In this regard, exercise has long been known to regulate metabolism, and has emerged as a promising therapy for management of autoimmune disorders. Hence, in this review, we inspect the role of key immunometabolic pathways specifically dysregulated in MS and highlight potential therapeutic benefits of exercise in modulating those pathways to harness an anti-inflammatory state. Finally, we touch upon current challenges and future directions for the field of exercise and immunometabolism in MS.
Keywords: CNS; EAE; amino acid; exercise; fatty acid metabolism; glycolysis; immunometabolism; mitochondria; multiple sclerosis; oxidative phosphorylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
-
Role of autophagy in the pathogenesis of multiple sclerosis.Neurosci Bull. 2015 Aug;31(4):435-44. doi: 10.1007/s12264-015-1545-5. Epub 2015 Aug 8. Neurosci Bull. 2015. PMID: 26254059 Free PMC article.
-
Alterations in Lymphocytic Metabolism-An Emerging Hallmark of MS Pathophysiology?Int J Mol Sci. 2023 Jan 20;24(3):2094. doi: 10.3390/ijms24032094. Int J Mol Sci. 2023. PMID: 36768415 Free PMC article. Review.
-
Progressive multiple sclerosis cerebrospinal fluid induces inflammatory demyelination, axonal loss, and astrogliosis in mice.Exp Neurol. 2014 Nov;261:620-32. doi: 10.1016/j.expneurol.2014.07.020. Epub 2014 Aug 8. Exp Neurol. 2014. PMID: 25111532
-
Physical Exercise Attenuates Experimental Autoimmune Encephalomyelitis by Inhibiting Peripheral Immune Response and Blood-Brain Barrier Disruption.Mol Neurobiol. 2017 Aug;54(6):4723-4737. doi: 10.1007/s12035-016-0014-0. Epub 2016 Jul 22. Mol Neurobiol. 2017. PMID: 27447807
Cited by
-
Response to physical activity of females with multiple sclerosis throughout the menstrual cycle: a protocol for a randomised crossover trial (EMMA Project).BMJ Open Sport Exerc Med. 2023 Nov 21;9(4):e001797. doi: 10.1136/bmjsem-2023-001797. eCollection 2023. BMJ Open Sport Exerc Med. 2023. PMID: 38022757 Free PMC article.
-
Mechanisms underlying the beneficial effects of physical exercise on multiple sclerosis: focus on immune cells.Front Immunol. 2023 Sep 29;14:1260663. doi: 10.3389/fimmu.2023.1260663. eCollection 2023. Front Immunol. 2023. PMID: 37841264 Free PMC article. Review.
-
Impact of Physical Activity on Cellular Metabolism Across Both Neurodegenerative and General Neurological Conditions: A Narrative Review.Cells. 2024 Nov 22;13(23):1940. doi: 10.3390/cells13231940. Cells. 2024. PMID: 39682689 Free PMC article. Review.
-
Upregulated expression of ubiquitin ligase TRIM21 promotes PKM2 nuclear translocation and astrocyte activation in experimental autoimmune encephalomyelitis.Elife. 2024 Sep 12;13:RP98181. doi: 10.7554/eLife.98181. Elife. 2024. PMID: 39264698 Free PMC article.
-
Application of eccentric training in various clinical populations: Protocol for a multi-centered pilot and feasibility study in people with low back pain and people with multiple sclerosis.PLoS One. 2022 Dec 22;17(12):e0270875. doi: 10.1371/journal.pone.0270875. eCollection 2022. PLoS One. 2022. PMID: 36548298 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

