Immune Checkpoints in Viral Infections
- PMID: 32967229
- PMCID: PMC7551039
- DOI: 10.3390/v12091051
Immune Checkpoints in Viral Infections
Abstract
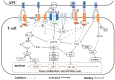
As evidence has mounted that virus-infected cells, such as cancer cells, negatively regulate the function of T-cells via immune checkpoints, it has become increasingly clear that viral infections similarly exploit immune checkpoints as an immune system escape mechanism. Although immune checkpoint therapy has been successfully used in cancer treatment, numerous studies have suggested that such therapy may also be highly relevant for treating viral infection, especially chronic viral infections. However, it has not yet been applied in this manner. Here, we reviewed recent findings regarding immune checkpoints in viral infections, including COVID-19, and discussed the role of immune checkpoints in different viral infections, as well as the potential for applying immune checkpoint blockades as antiviral therapy.
Keywords: chronic infection; immune checkpoint; immunotherapy; virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Cancer immunotherapy by targeting immune checkpoints: mechanism of T cell dysfunction in cancer immunity and new therapeutic targets.J Biomed Sci. 2017 May 25;24(1):35. doi: 10.1186/s12929-017-0341-0. J Biomed Sci. 2017. PMID: 28545567 Free PMC article. Review.
-
The role of IL-10 in regulating immunity to persistent viral infections.Curr Top Microbiol Immunol. 2011;350:39-65. doi: 10.1007/82_2010_96. Curr Top Microbiol Immunol. 2011. PMID: 20703965 Free PMC article. Review.
-
The Era of Immune Checkpoint Therapy: From Cancer to Viral Infection-A Mini Comment on the 2018 Medicine Nobel Prize.Virol Sin. 2018 Dec;33(6):467-471. doi: 10.1007/s12250-018-0077-3. Epub 2018 Dec 20. Virol Sin. 2018. PMID: 30570713 Free PMC article. No abstract available.
-
Viral interference and interferon.Ric Clin Lab. 1975 Jul-Sep;5(3):196-213. doi: 10.1007/BF02908284. Ric Clin Lab. 1975. PMID: 778995 Review.
-
Viral pathogenesis, modulation of immune receptor signaling and treatment.Adv Exp Med Biol. 2008;640:325-49. doi: 10.1007/978-0-387-09789-3_22. Adv Exp Med Biol. 2008. PMID: 19065800 Free PMC article. Review.
Cited by
-
Hydroxyproline metabolism enhances IFN-γ-induced PD-L1 expression and inhibits autophagic flux.Cell Chem Biol. 2023 Sep 21;30(9):1115-1134.e10. doi: 10.1016/j.chembiol.2023.06.016. Epub 2023 Jul 18. Cell Chem Biol. 2023. PMID: 37467751 Free PMC article.
-
Enhanced Anti-Tumor Response Elicited by a Novel Oncolytic Pseudorabies Virus Engineered with a PD-L1 Inhibitor.Viruses. 2024 Jul 31;16(8):1228. doi: 10.3390/v16081228. Viruses. 2024. PMID: 39205202 Free PMC article.
-
Up-Regulation of Immune Checkpoints in the Thymus of PRRSV-1-Infected Piglets in a Virulence-Dependent Fashion.Front Immunol. 2021 May 11;12:671743. doi: 10.3389/fimmu.2021.671743. eCollection 2021. Front Immunol. 2021. PMID: 34046040 Free PMC article.
-
Predicting survival in patients with SARS-CoV-2 based on cytokines and soluble immune checkpoint regulators.Front Cell Infect Microbiol. 2024 Nov 25;14:1397297. doi: 10.3389/fcimb.2024.1397297. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39654974 Free PMC article.
-
Modulating Immune Response in Viral Infection for Quantitative Forecasts of Drug Efficacy.Pharmaceutics. 2023 Jan 3;15(1):167. doi: 10.3390/pharmaceutics15010167. Pharmaceutics. 2023. PMID: 36678799 Free PMC article. Review.
References
-
- DALYs G.B.D., Collaborators H. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet (Lond. Engl.) 2017;390:1260–1344. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

