NRF2 Is an Upstream Regulator of MYC-Mediated Osteoclastogenesis and Pathological Bone Erosion
- PMID: 32967239
- PMCID: PMC7564846
- DOI: 10.3390/cells9092133
NRF2 Is an Upstream Regulator of MYC-Mediated Osteoclastogenesis and Pathological Bone Erosion
Abstract
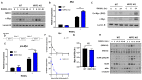
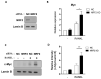
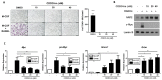
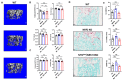
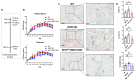
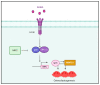
Osteoclasts are the sole bone-resorbing cells that play an essential role in homeostatic bone remodeling and pathogenic bone destruction such as inflammatory arthritis. Pharmacologically targeting osteoclasts has been a promising approach to alleviating bone disease, but there remains room for improvement in mitigating drug side effects and enhancing cell specificity. Recently, we demonstrated the crucial role of MYC and its downstream effectors in driving osteoclast differentiation. Despite these advances, upstream regulators of MYC have not been well defined. In this study, we identify nuclear factor erythroid 2-related factor 2 (NRF2), a transcription factor known to regulate the expression of phase II antioxidant enzymes, as a novel upstream regulator of MYC. NRF2 negatively regulates receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclastogenesis through the ERK and p38 signaling-mediated suppression of MYC transcription. Furthermore, the ablation of MYC in osteoclasts reverses the enhanced osteoclast differentiation and activity in NRF2 deficiency in vivo and in vitro in addition to protecting NRF2-deficient mice from pathological bone loss in a murine model of inflammatory arthritis. Our findings indicate that this novel NRF2-MYC axis could be instrumental for the fine-tuning of osteoclast formation and provides additional ways in which osteoclasts could be therapeutically targeted to prevent pathological bone erosion.
Keywords: MYC; NRF2; RANKL signaling; osteoclasts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

