Characterization of Tauopathy in a Rat Model of Post-Stroke Dementia Combining Acute Infarct and Chronic Cerebral Hypoperfusion
- PMID: 32967251
- PMCID: PMC7555397
- DOI: 10.3390/ijms21186929
Characterization of Tauopathy in a Rat Model of Post-Stroke Dementia Combining Acute Infarct and Chronic Cerebral Hypoperfusion
Abstract
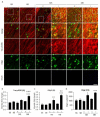
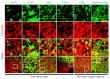
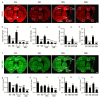
Post-stroke dementia (PSD) is a major neurodegenerative consequence of stroke. Tauopathy has been reported in diverse neurodegenerative diseases. We investigated the cognitive impairment and pathomechanism associated with tauopathy in a rat model of PSD by modeling acute ischemic stroke and underlying chronic cerebral hypoperfusion (CCH). We performed middle cerebral artery occlusion (MCAO) surgery in rats to mimic acute ischemic stroke, followed by bilateral common carotid artery occlusion (BCCAo) surgery to mimic CCH. We performed behavioral tests and focused on the characterization of tauopathy through histology. Parenchymal infiltration of cerebrospinal fluid (CSF) tracers after intracisternal injection was examined to evaluate glymphatic function. In an animal model of PSD, cognitive impairment was aggravated when BCCAo was combined with MCAO. Tauopathy, manifested by tau hyperphosphorylation, was prominent in the peri-infarct area when CCH was combined. Synergistic accentuation of tauopathy was evident in the white matter. Microtubules in the neuronal axon and myelin sheath showed partial colocalization with the hyperphosphorylated tau, whereas oligodendrocytes showed near-complete colocalization. Parenchymal infiltration of CSF tracers was attenuated in the PSD model. Our experimental results suggest a hypothesis that CCH may aggravate cognitive impairment and tau hyperphosphorylation in a rat model of PSD by interfering with tau clearance through the glymphatic system. Therapeutic strategies to improve the clearance of brain metabolic wastes, including tau, may be a promising approach to prevent PSD after stroke.
Keywords: animal model; chronic cerebral hypoperfusion; glymphatic system; post-stroke dementia; tauopathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

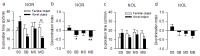

Similar articles
-
Chronic cerebral hypoperfusion induces post-stroke dementia following acute ischemic stroke in rats.J Neuroinflammation. 2017 Nov 9;14(1):216. doi: 10.1186/s12974-017-0992-5. J Neuroinflammation. 2017. PMID: 29121965 Free PMC article.
-
Chronic Cerebral Hypoperfusion Aggravates Parkinson's Disease Dementia-Like Symptoms and Pathology in 6-OHDA-Lesioned Rat through Interfering with Sphingolipid Metabolism.Oxid Med Cell Longev. 2022 Aug 8;2022:5392966. doi: 10.1155/2022/5392966. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35979400 Free PMC article.
-
Prolonged Chronic Cerebral Hypoperfusion Does not Exacerbate Tau Pathology in a Tauopathy Mouse Model.J Integr Neurosci. 2025 Feb 21;24(2):26108. doi: 10.31083/JIN26108. J Integr Neurosci. 2025. PMID: 40018776
-
Rodent Models of Post-Stroke Dementia.Int J Mol Sci. 2022 Sep 15;23(18):10750. doi: 10.3390/ijms231810750. Int J Mol Sci. 2022. PMID: 36142661 Free PMC article. Review.
-
Chronic Traumatic Encephalopathy: Is Latency in Symptom Onset Explained by Tau Propagation?Cold Spring Harb Perspect Med. 2018 Feb 1;8(2):a024059. doi: 10.1101/cshperspect.a024059. Cold Spring Harb Perspect Med. 2018. PMID: 28096246 Free PMC article. Review.
Cited by
-
Neuroinflammation as a Key Driver of Secondary Neurodegeneration Following Stroke?Int J Mol Sci. 2021 Dec 3;22(23):13101. doi: 10.3390/ijms222313101. Int J Mol Sci. 2021. PMID: 34884906 Free PMC article. Review.
-
Deciphering aquaporin-4's influence on perivascular diffusion indices using DTI in rat stroke studies.Front Neurosci. 2025 Aug 5;19:1566957. doi: 10.3389/fnins.2025.1566957. eCollection 2025. Front Neurosci. 2025. PMID: 40837880 Free PMC article.
-
Participation of Amyloid and Tau Protein in Post-Ischemic Neurodegeneration of the Hippocampus of a Nature Identical to Alzheimer's Disease.Int J Mol Sci. 2021 Feb 28;22(5):2460. doi: 10.3390/ijms22052460. Int J Mol Sci. 2021. PMID: 33671097 Free PMC article. Review.
-
The mechanisms underlying the actions of Xuefu Zhuyu decoction pretreatment against neurological deficits after ischemic stroke in mice: The mediation of glymphatic function by aquaporin-4 and its anchoring proteins.Front Pharmacol. 2022 Dec 13;13:1053253. doi: 10.3389/fphar.2022.1053253. eCollection 2022. Front Pharmacol. 2022. PMID: 36582539 Free PMC article.
-
Impaired cerebrospinal fluid circulation and cerebral lymphatic drainage in a rat model of chronic hydrocephalus.Front Mol Neurosci. 2025 Feb 19;18:1516265. doi: 10.3389/fnmol.2025.1516265. eCollection 2025. Front Mol Neurosci. 2025. PMID: 40046632 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

